INTRODUCTION
With the development of life and its complexities, and the expansion of scientific progress and inventions that are made to serve humans, it becomes necessary to study the effects and reflections of some of these advances affecting negatively the nature and humans. Here, we discuss the accomplishments of devices that work with electromagnetic waves, thus affecting human life one way or the other. However, electromagnetic waves in the fields of energy and communication, directly or indirectly, have affected human health.1 Visible light, microwaves, x-rays, gamma radiations, and radio and television are known electromagnetic devices that have the same properties but differ in wavelength and frequency. Electromagnetic field (EMF) consists of vibrating electric and magnetic fields that are perpendicular to each other; these fields are described in terms of magnitude and direction. These electric and magnetic fields interact directly with biological systems and affect living cells in humans, animals as well as plants.1 Electromagnetic waves cause biological changes that sometimes, but not always, lead to harmful effects on health.2 Biological effects take place when the body is exposed to electromagnetic waves that lead to significant or detectable physiological changes in the system. The harmful effects on health appear if the body is not able to resist the same.2 When studying the mutual effects of EMFs on the surfaces exposed to them, we use the concept of power density, which is estimated in watts per square meter (w/m2). Power density is inversely proportional to square of the distance between the source of electromagnetic waves and the receiving surface. The flowing power takes its highest value near antenna and decreases as one moves away from it, so that the strength of EMF gets inversely proportional to the square dimension.4,3 On the biological side, the interaction of EMFs with a living organism varies according to the frequency used and the biological nature of the exposed medium. Several studies have demonstrated different effects of EMFs. Exposure to a particular intensity of these fields leads to chemical and biological changes in the body that could be in the form of burns or injury to the eye if exposed to visual cataract.5 The path depth of EMFs inside a living body decreases with increasing frequency. In order to understand the mechanism of mutual influence between EMF and the body, we must consider differences between structural and electrical properties of various parts of the body. The effect of EMF varies according to its frequency. There are ions or substances of polar nature that make changes in the exposed medium. This is in addition to materials that have magnetic features that make them more sensitive to the applied external field, such as some iron oxides. Presence of ions in the exposed body increases the mutual effect between the applied EMF and medium and the rate of specific energy absorbed.6 Some studies have indicated that these waves have thermal and biological effects that cause insomnia, headache, and temporary memory loss. These effects depend on the frequency of waves and the amount of energy absorbed by body tissues, in addition to the length of exposure to these waves.7 Recent studies have established that electromagnetic waves contribute to the development of free radicals inside cells by disrupting the action of natural antioxidants.6,8
MATERIALS AND METHODS
Sample Collection
The current study involved Internet network stations in the Samarra city of Iraq. A questionnaire was distributed to persons exposed to the radioactive frequencies of Internet towers. The questionnaire included name, age, gender, and period of exposure. Samples were taken from subjects having no chronic disease to avoid interference with the results of the present research, and those who were exposed to radiations of the Internet towers for 1–10 years. The study comprised samples collected from 43 exposed and 20 non-exposed males and females, and the age of participants ranged from 20 to 35 years.
A disposable syringe was used to take 5-mL blood from each participant, and all blood samples were collected in anticoagulant-free gel tubes. The samples were centrifuged at 3500 rpm for 10 min to obtain blood serum. The serum was divided into five groups in small tubes. All serum samples were kept in boxes and stored at freezing temperature until biochemical tests were performed for antioxidants.9–12
Statistical Analysis
Mean values and standard deviation (SD) were calculated, and Student’s t-test was used to compare the results of different groups.12
RESULTS AND DISCUSSION
Data Description
The collected data were put in common statistical tables using the Student’s t-test. Data features were highlighted in either simple or double frequency tables. The data were represented graphically to calculate statistical measures that highlighted its character. The frequencies and percentage values of all variables were obtained for the following dispersions.
Gender
Table 1 shows the distribution of participants according to gender. The study comprised 57% males, which was the highest proportion, and 43% females, being the lowest proportion.
TABLE 1. Distribution of Collected Samples According to Gender.
| Gender | Frequencies | Percentage (%) |
|---|---|---|
| Male | 24 | 57 |
| Female | 18 | 43 |
| Total | 42 | 100 |
A graph of circular sectors was used to describe the percentage of male and female participants in the study as shown in Figure 1.
FIGURE 1. The percentage of male and female participants in the study.
Age
Table 2 shows the distribution of study participants according to age. We observed that maximum participants (34.8%) were in the age group of 20–25 years, and minimum participants (27.9%) were in the age group of 25–30 years.
TABLE 2. Distribution of Participants According to Age.
| Age range (in years) | Numbers | Percentage (%) |
|---|---|---|
| 20–25 | 15 | 34.8 |
| 25–30 | 12 | 27.9 |
| 30–35 | 13 | 30.2 |
| Total | 40 | 100 |
Graphically, the age distribution of study participants is shown in Figure 2.
FIGURE 2. The age distribution of study samples.
Exposure Period
Table 3 shows the distribution of study samples according to the period of exposure. It was observed that the highest vulnerability to radiations (83%) was in those exposed for 1–5 years, and the lowest percentage (11.6%) was in those exposed for 5–10 years.
TABLE 3. Distribution of Participants According to Exposure Period.
| Exposure period (years) | Numbers | Percentage (%) |
|---|---|---|
| 1–5 | 36 | 83 |
| 5–10 | 05 | 11.6 |
| Total | 41 | 94.6 |
Graphically, the distribution of exposure period for study participants is shown in Figure 3.
FIGURE 3. Distribution of exposure period for study participants.
Determination of antioxidants in serum samples of participants exposed to electromagnetic waves of Internet towers
Table 4 shows the mean ± SD values of antioxidant level represented by biochemical measurements of glutathione peroxidase (GPx), superoxide dismutase (SOD), glutathione (GSH), peroxynitrate (ONOO), and malondialdehyde (MDA) in the serum samples of exposed participants.
TABLE 4. Levels of glutathione peroxidase (GPx), superoxide dismutase (SOD), glutathione (GSH), peroxynitrate (ONOO-), and malonaldehyde (MDA) in the serum samples of participants exposed to electromagnetic waves.
| Antioxidants | Mean ± SD | |
|---|---|---|
| Control (non-exposed) group | Exposed group | |
| GPx (U/L) | 2.724 ± 0.682 | 4.509 ± 2.035 |
| SOD (U/L) | 1.285 ± 0.554 | 6.260 ± 7.471 |
| GSH (μmol/L) | 0.081 ± 0.03 | 0.0708 ± 0.0451 |
| Peroxynitrate (μmol/L) | 0.360 ± 0.246 | 0.660 ± 0.3081 |
| MDA (μmol/L) | 2.14 ± 0.804 | 12.670 ± 3.919 |
Glutathione Peroxidase
The efficacy of glutathione peroxidase was calculated for the serum samples of participants exposed to electromagnetic waves and control group comprising non-exposed participants. The mean ± SD values of both groups are shown in Table 4 and Figure 4.
FIGURE 4. Modifying the activity of glutathione peroxidase (U/L) in the serum samples of studied groups.
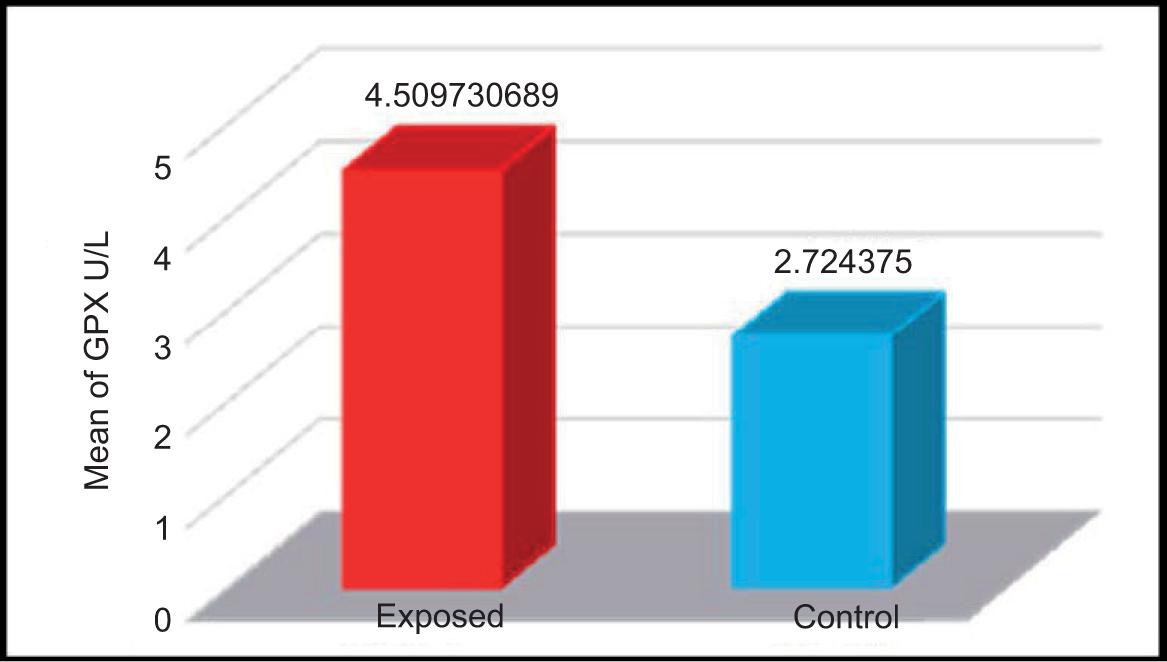
The mean ± SD of the control group and the exposed group were 2.724 ± 0.682 U/L and 4.509 ± 2.035 U/L, respectively. The results of the present study indicated a significant increase in the concentration of glutathione peroxidase in the serum of the subjects exposed to electromagnetic waves compared to the control group of non-exposed participants. This result contravenes results of the study conducted by Kumari et al.13 that found decrease of the enzyme in male rats exposed to electromagnetic radiation of mobile phones and microwaves for 2 h a day for 35 days.
Superoxide dismutase
The efficacy of superoxide dismutase enzyme was calculated in the serum samples of both exposed and non-exposed participants. The mean ± SD values of both groups are shown in Table 4 and Figure 5.
FIGURE 5. Activity of superoxide dismutase enzyme (U/L) in the serum samples of studied groups.
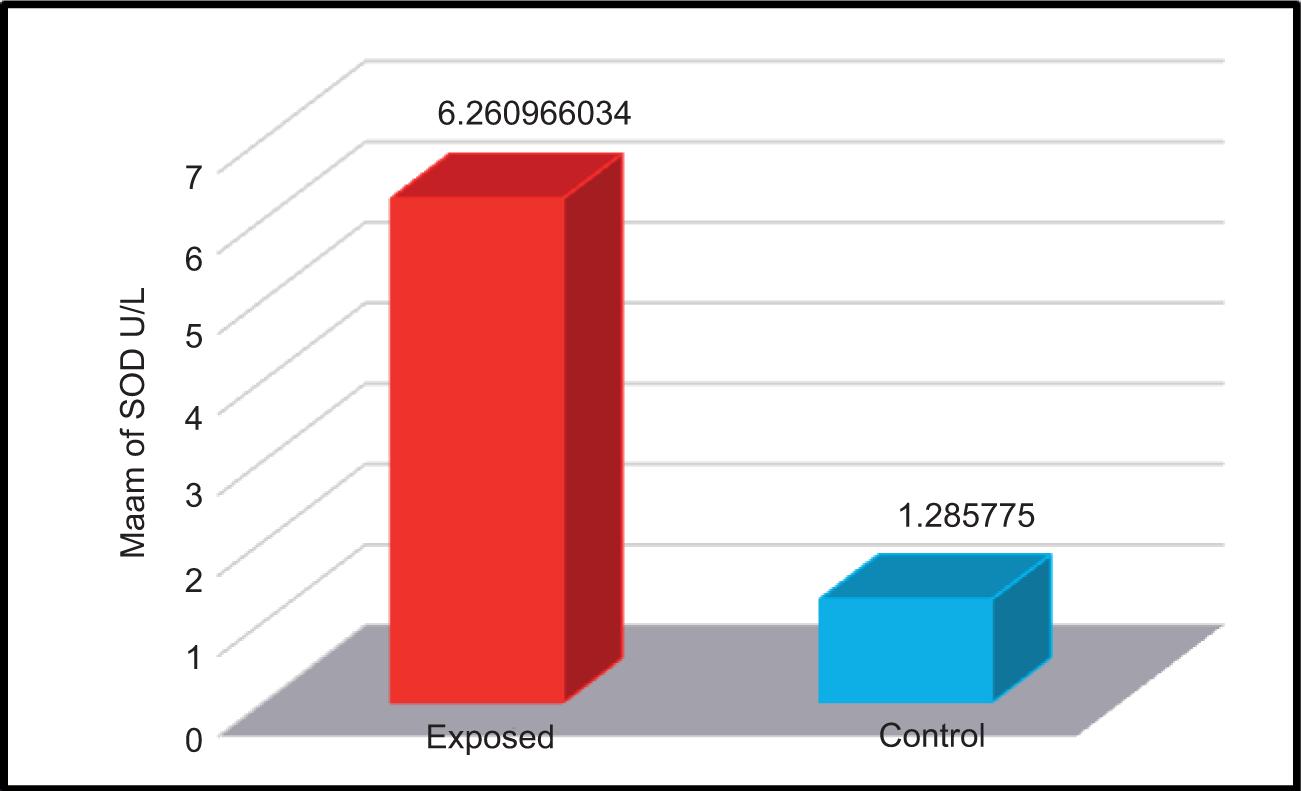
The mean ± SD values of the control and exposed groups were 1.285 ± 0.554 U/L and 6.260 ± 7.471 U/L, respectively. The results indicated a significant increase in the concentration of SOD enzyme in the serum samples of participants exposed to electromagnetic waves compared to that of non-exposed participants. These results contravene the results of the studies conducted by Kumari et al.13 and Sepehrimanesh et al.14 They studied the effect of 900-MHz EMF on the antioxidant enzymes of mice serum for 30 days, and observed decrease in both GPx and SOD activities.14
Glutathione
The concentration of glutathione was measured in the serum samples of both exposed and non-exposed groups. The mean ± SD values of both groups are shown in Table 4 and Figure 6.
FIGURE 6. Glutathione levels (mM/L) in the serum samples of studied groups.
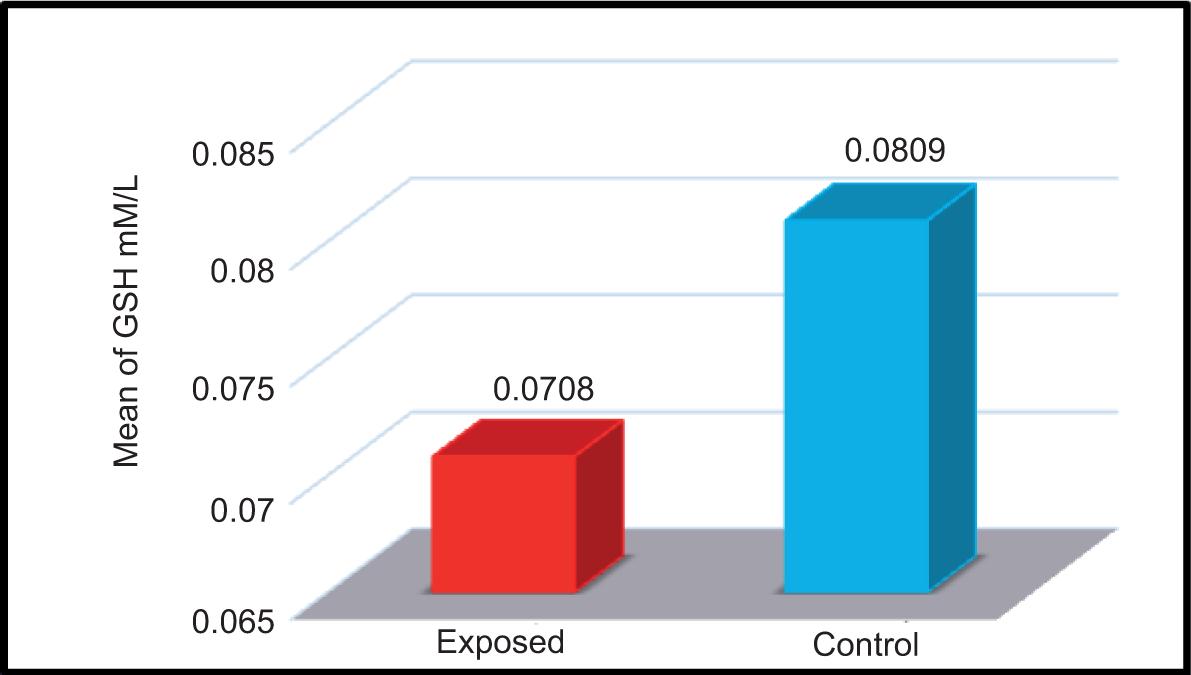
The mean ± SD of the control and exposed groups were 0.081 ± 0.03 mM/L and 0.0708 ± 0.0451 mM/L, respectively. The results of the current study demonstrated a significant decrease in GSH levels in the serum samples of participants exposed to electromagnetic waves compared to that of non-exposed participants. The results of the current study agreed with the results of Arendash et al.15 and Meral et al.,16 which showed that exposure to EMFs led to a decrease in the levels of GHS in mice and guinea pigs.
Peroxynitrate
The concentration of peroxynitrate was measured in the serum samples of participants exposed and not exposed to electromagnetic waves. The mean ± SD values of both groups are shown in Table 4 and Figure 7.
FIGURE 7. Levels of peroxynitrate (mM/L) in the serum samples of studied groups.
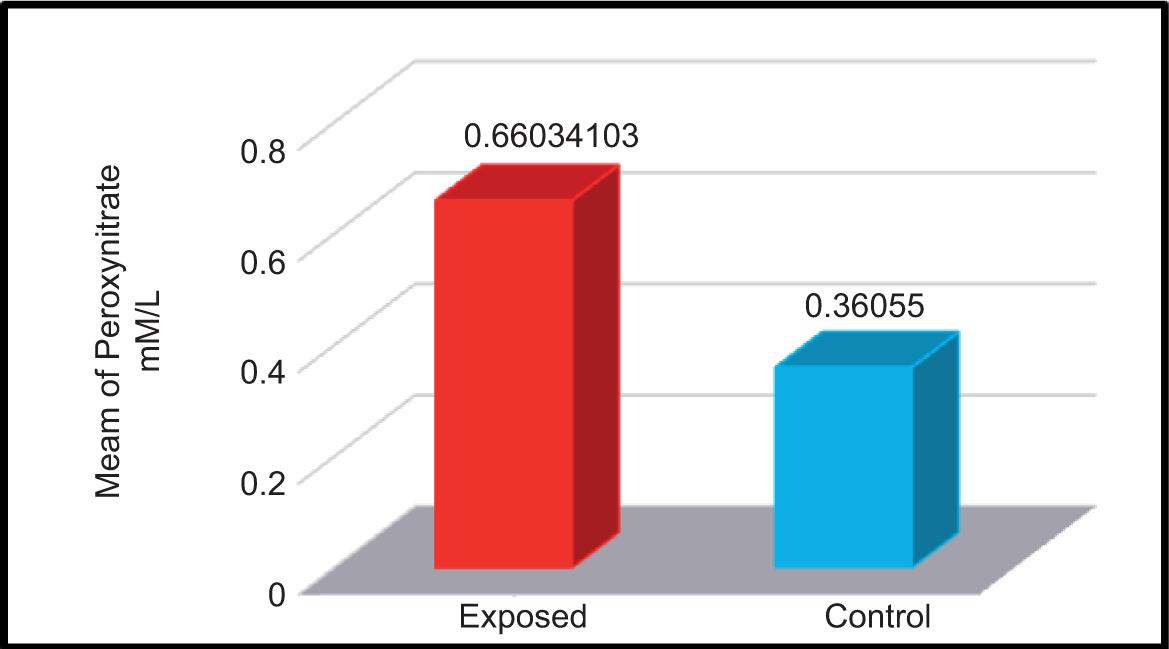
The mean ± SD values of both control and exposed groups were 0.3605 ± 0.246 mM/L and 0.6603 ± 0.3081 mM/L, respectively. The enzyme increased in the serum of subjects exposed to electromagnetic waves compared to the control group comprising non-exposed participants. In their study, Kivrak et al.17 had proved that exposure to EMFs, similar to other stress factors, resulted in oxidative stress through an increase in lipid and protein oxidation in body tissues, in addition to significant changes in the levels of GSH, GPx, SOD, and catalase (CAT).
Malondialdehyde
The concentration of MDA was measured in the serum samples of both exposed and non-exposed participants. The mean ± SD values of both groups are shown in Table 4 and Figure 8.
FIGURE 8. Levels of malondialdehyde (mM/L) in the serum samples of studied groups.
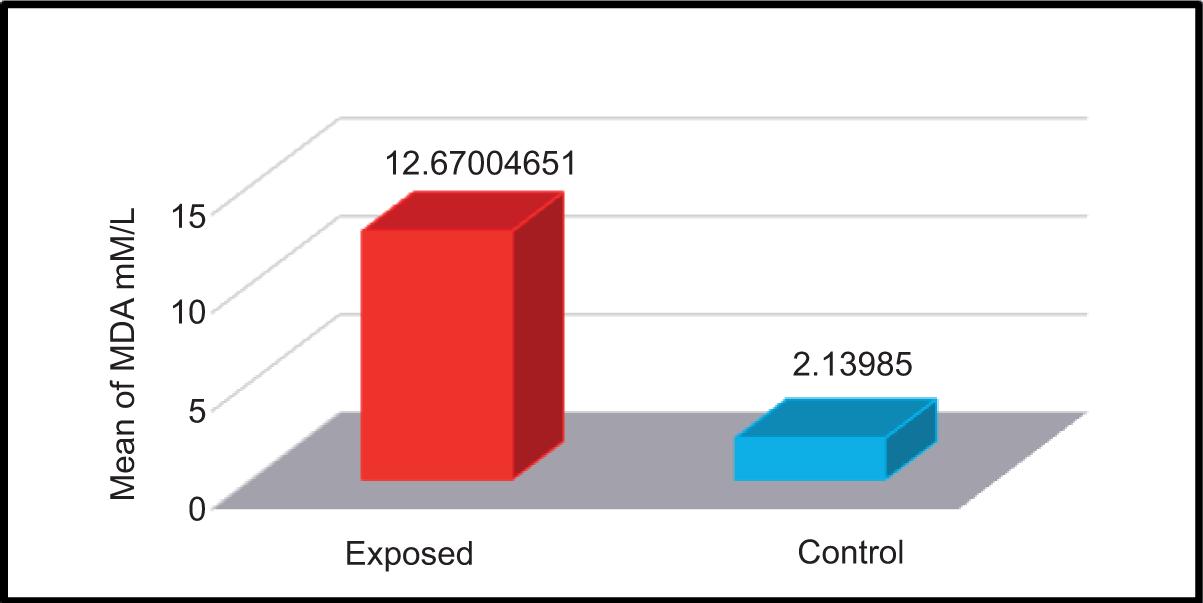
The mean ± SD of both control and exposed groups were 2.14 ± 0.804 mM/L and 12.670 ± 3.919 mM/L, respectively. Results of the current study showed a significant increase in the concentration of MDA in the serum samples of participants exposed to electromagnetic waves compared to the control group of non-exposed participants. These results were consistent with the findings of Kumari et al.13 They observed an increase in the efficacy of MDA level when male rats were exposed to the electromagnetic radiations of mobile phones and microwave devices daily for 2 h for 35 days. The study conducted by Ghanbari et al. showed that 50-day exposure to EMF caused oxidative stress by increasing both MDA levels and GPx activity and decreasing SOD activity.18
A number of studies have been conducted related to the nonthermal effects of radiofrequencies of EMF on biological tissues.19 It has been observed that this effect mediates the generation of reactive oxygen species (ROS).20 ROS participates in various cellular functions that are necessary but highly toxic to cellular homeostasis.21 Its cytotoxic effects result from the oxidation of phospholipids, which changes membrane conductivity and a loss of cell membrane integrity.22 Several studies have confirmed that exposure to EMFs increases the production of free radicals in living cells. Living organisms have antioxidant mechanisms of GSH, GPx, SOD, and CAT. These antagonists function to reduce the damage caused by free radical ROS and their products.23 This defense mechanism works by suppressing or blocking chain reactions produced by free radicals. Exposure to factors that cause excessive production of free radical ROS, including EMFs, leads to oxidative stress.24 Studies in the recent years have indicated that free radicals play a major role in the development of many diseases such as cancer and diabetes.25
AUTHOR DECLARATION
The manuscript was written in original, and all the data and results pertaining to this study were original according to the performed research. The authors followed academic integrity and had not copied any content/results from other sources.