INTRODUCTION
Toxoplasmosis is a zoonotic, foodborne disease caused by an obligate intracellular parasite called Toxoplasma gondii.1–4 Globally, it is one of the most prevalent zoonotic diseases that highly affects a wide range of populations and is endemic in many areas.5–7 The effect of toxoplasmosis on public health is significantly evident worldwide.1–4 Humans acquire the infection in different ways: by consuming raw or undercooked infected meat with tissue cysts of the parasite, by ingesting food and drinks contaminated with the parasite’s oocysts. Humans can also passively acquire the tachyzoite stage transplacentally, or by the transfusion of the blood and its products, and also via organ transplantation.8–11
The parasite develops in different sites in the body of the infected individuals, induces various pathological changes displayed in numerous patterns which results in various clinical manifestations.12 Although acute infection is typically asymptomatic, 10–20% of the patients develop bilateral, cervical, or axillary lymphadenopathy.13–15 During the chronic phase of the disease, the parasite encrypts within the tissue cysts that develop in different tissue types in the patient’s body, particularly in the brain, liver, and muscles, whenever the disease transforms from acute to the chronic phase.16–18 These tissue cysts have the potential ability to remain in the intermediate host for their entire life.17,19
In the infected humans, the Toxoplasma parasite directly triggers a robust immune response against the spreading parasite, including both cellular and humoral elements.20 This activation of the immune system enhances the parasite to hide in a more tolerant form where it transforms from tachyzoites to bradyzoites inside the host’s cell, along with consequent differentiation in the host cell to form a cyst (zoitocyst) in the tissue, as found in the latent infection stage.21,22 The parasite transforms from the zoitocyst stage to the tachyzoite stage in immunocompromised individuals such as AIDS patients. Similarly, congenitally infected patients may experience multiple reactivations. The interconversion between the tachyzoites and bradyzoites is critical not only for the establishment of the chronic infection but also for disease recurrence.23,24
Nevertheless, cell-mediated immunity (CMI) plays an active role in driving the encystment of this parasite. Both CD4+ and CD8+ cells can lyse the infected cells with T. gondii.25,26 These T-cell subsets synergize with NK, macrophages, and lymphokine-activated killer cells in the protective mechanisms of CMI.26 In addition, the infection causes the release of different cytokines and pro-inflammatory mediators from the activated T-helper cells.25 However, T-lymphocytes are the source of these cytokines during the chronic phase.27,28
Moreover, the primary infection with T. gondii induces the production of different immunoglobulin isotypes, which appear sequentially with various kinetics.29,30 It has been found that Toxoplasma infection amplifies the levels of the specific circulating anti-Toxoplasma immunoglobulins IgA, IgM, IgE, and IgG.31,32 The IgM and IgA appear during the first week and reach their highest levels after approximately 30 days before declining to undetectable levels after several weeks to years.33 Whereas the first detection of the IgG is evident 14 days after IgM appearance and reaches a plateau after around 2−3 months following the infection, which then steadily declines to a residual lifelong titer.25,33 However, the quantitative, qualitative, and kinetic analyses of the antibodies, mostly the IgM and IgG, have a substantial value in the diagnosis, monitoring, and epidemiological studies of toxoplasmosis.34,35 While detecting the specific IgM of Toxoplasma refers to a new exposure or ongoing active infection, the IgM antibodies might be long-lasting for more than 18 months after the primary exposure to T. gondii.36 On the other hand, the elevation of the specific Toxoplasma IgG may last for many years after the primary infection, although it may indicate a secondary infection with the parasite.37 The primary infection is confirmed if the specific Toxoplasma IgG appeared after the IgM. While chronic (ongoing) toxoplasmosis is usually diagnosed by detecting the anti-Toxoplasma IgG in the sera of the infected individuals,31 detecting the IgM and/ or the IgA antibodies usually refers to the acute phase of the disease.38
Chitinase-3-like protein 1 or YKL-40 is a glycoprotein that belongs to the glycoside hydrolase family 18. A range of cells are responsible for the synthesis, secretion, and regulation of YKL-40, such as the macrophage, neutrophils, chondrocytes, synoviocytes, tumor cells,39–41 smooth muscle cells (SMC), hepatic stellate cells (HSC), and fibroblast-like cells (FLC).39,40,42 Its expression is controlled by miRNAs, cytokines, growth factors (GF), drugs, and stress. The YKL-40 has important functions in many biological processes. It plays a major role in the inflammatory reactions, tissue injury, remodeling, and repair,43 and has a strong correlation with many diseases.44 However, it is still unknown whether YKL-40 is markedly secreted in Toxoplasmosis or not and whether its level is different in the acute or chronic stage of the infection. Therefore, this study aimed to determine whether YKL-40 is markedly increased in toxoplasmosis or not and whether the level is different between the acute and chronic phases of the infection to determine if it can be used as a clinically useful biomarker in the diagnosis, determination of disease severity, and follow-up of toxoplasmosis.
MATERIALS AND METHODS
Samples collection
A total of 80 serum samples were collected from previously diagnosed female patients with toxoplasmosis, who were of different ages, and they did not complain of any other diseases. In addition, 10 healthy females were used as controls. Yet, the IgM and IgG tests were repeated for them to confirm and differentiate between the acute and chronic infections. Serum samples were collected from July 2020 to February 2021 from the Department of Gynecology in Al-Elwiyah and Kamal Al-Samarrai Hospitals in Baghdad, Bint Al-Huda Teaching Hospital, Al-Shatrah General Hospital in Dhi-Qar, Babil Teaching Hospital for Maternity and Children in Babil, and Basrah General Hospital in Al-Basrah, in addition to Ur Private Gynecology clinics in Dhi-Qar, Ozone Private Medical Laboratory, Ibn Al-Bitar laboratory in Dhi-Qar, and Maysan Medical Laboratory in Maysan.
A total of 5 mL of venous blood was collected from the subjects and the control in gel tubes, and centrifuged for 5 min at 5000 rpm. The serum was collected and stored at −20°C until use. Finally, samples were tested using the serological testing kits: Anti-Toxoplasma IgM EIA, Anti-Toxoplasma IgG EIA Kits (Foresight ACON Laboratories, USA), and Human CHI3L1 ELISA Kit (MyBiosource, USA). Tests were performed according to the manufacturer’s instructions in each kit.
Data management and analysis
Statistical analysis of data was performed using SPSS software (version 25.0). Descriptive statistics (frequencies and percentages) were used to summarize the dependent and resulting variables. The value of P = 0.05 was considered statistically significant.
RESULTS
IgM and IgG levels
The first step performed after the collection of serum samples was a confirmatory investigation on the immunoglobulin status of the basic two types of anti-Toxoplasma antibodies, IgM and IgG, in the patients and control sera to make sure that toxoplasmosis infection is present and whether they have an acute or chronic infection to distribute the collected samples into two groups for the later work. The levels of the specific anti-Toxoplasma IgM and IgG antibodies were adopted for this purpose.
It was reported that 30 patients were IgM+ve, 29 (97%) were IgG-ve, and only one patient was IgG+ve. While the remaining 50 patients were IgG+ve and IgM-ve, and all the 10 control individuals (100%) were IgM-ve and IgG-ve. Accordingly, the patients were divided into two groups, acute and chronic infection groups. The level of these two basic types of immunoglobulins prominently varied within the analyzed samples of the infected group, where the level of each immunoglobulin was very highly significantly different (P ≤ 0.001) between the two types of infection.
The level of YKL-40
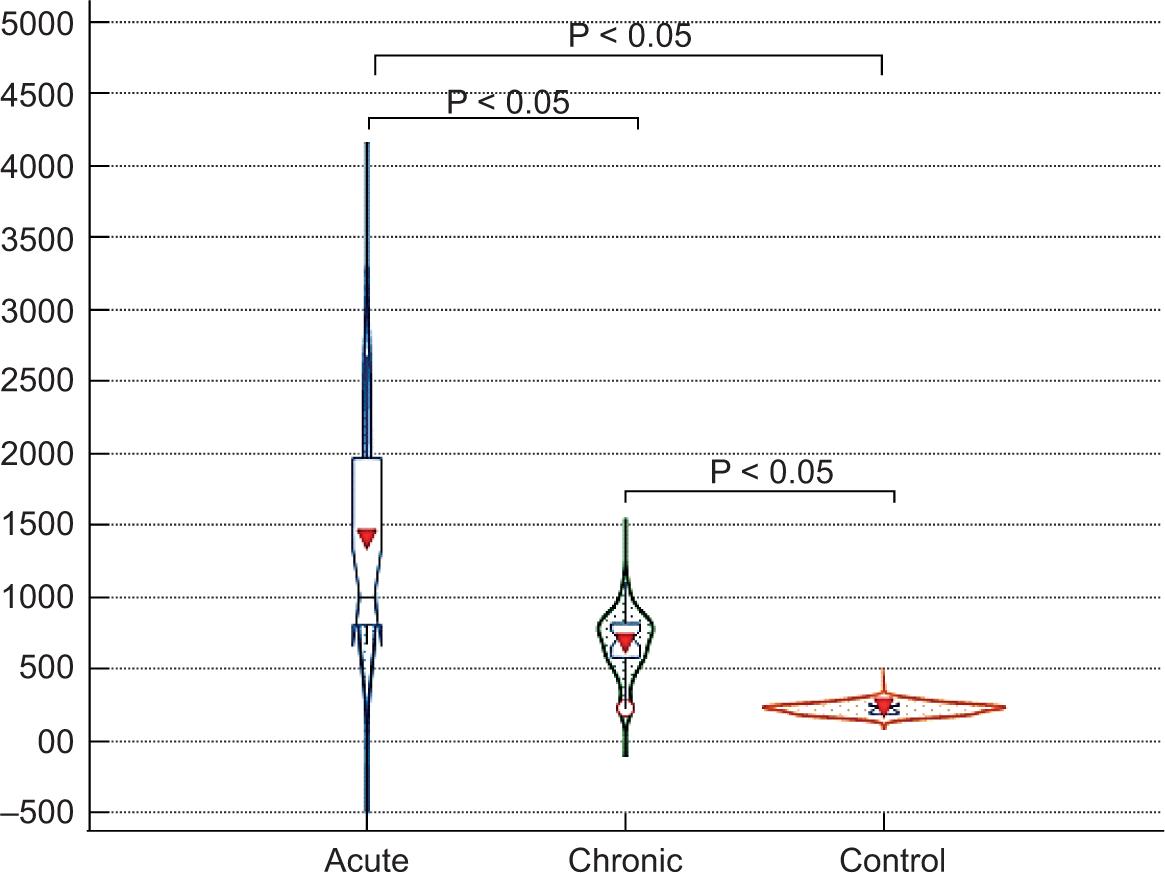
The results of the YKL-40 test showed that there is a very highly significant difference in the level of this marker among the acute, chronic, and control groups (P < 0.001). The median level of the YKL-40 in the group of the acute infection was 989.39 pg/mL, while in the group of chronic infection it was 744.30 pg/mL, and in the control group it was 235.22 pg/mL (Table 1).
TABLE 1. The level of YKL-40 in the acute, chronic, and control groups according to Kruskal–Wallis test.
| Variable | Groups | Median pg/mL | 1st IQR to 3rd IQR | P |
|---|---|---|---|---|
| YKL-40 pg/mL | Acute | 989.39 | 816.67–1909.58 | <0.001 |
| Chronic | 744.30 | 581.48–844.99 | ||
| Control | 235.22 | 203.98–274.34 |
IQR, interquartile range; SD, standard deviation; No, number and significance at P < 0.05.
Pairwise comparisons were used to reach the significant difference between each pair of groups. The results of the multiple comparisons showed very highly significant differences in the level of YKL-40 based on a P < 0.001 between the pairs Acute–Chronic, Acute–Control, and Chronic–Control. Based on the data, it has been observed that the level of YKL-40 in the acute infection group was very highly significantly different from its level in the chronic infection and control groups, while its level was much higher in the group of the acute infection, as presented in Figure 1.
FIGURE 1. The YKL-40 levels in the studied groups by violin and box plot comparison. The arrowhead in the middle is the mean value. Error bar is one SD. The blue box represents the interquartile range (IQR), the horizontal line in the middle is the Median. The shape of the violin displays frequencies of observations.
The receiver operating characteristic (ROC) analysis was used in the estimation of the sensitivity and specificity of the cutoff value of the YKL-40, at which the patients considered infected with acute or chronic toxoplasmosis if its value was higher than the specified cutoff value of this parameter. The comparison of the ROC curve and functional area under the curve (AUC) between the acute toxoplasmic patients and healthy individuals (controls) elucidated that YKL-40 is an excellent parameter, where the ROC curve showed that the AUC of YKL-40 is 1.00 and the cutoff value is >669.078. The sensitivity and specificity were 100, and therefore, the YKL-40 can be considered a very highly sensitive biomarker (P < 0.0001) in the detection of the acute toxoplasmosis (Table 2 and Figure 2a).
TABLE 2. The ROC analysis criteria of YKL-40.
| Parameter | Comparation | AUC | SE* | 95% CI** | Cutoff | Se. | Sp. | P |
|---|---|---|---|---|---|---|---|---|
| YKL-40 | Acute infection vs Control | 1.00 | 0.00 | 0.912–1.000 | >669.078 | 100 | 100 | <0.0001 |
| Chronic infection vs Control | 0.898 | 0.051 | 0.792–0.961 | >283.563 | 92 | 80 | <0.0001 |
**Binomial exact. AUC, area under curve; CI, confidence interval; P, level of P significance (P < 0.05); ROC, receiver operating characteristic; SE, standard error; Se, sensitivity; Sp, specificity.
FIGURE 2. Plot of ROC curve analysis of YKL-40. (A) Identification of acute toxoplasmosis patients from healthy controls; (B) Identification of chronic toxoplasmosis patients from healthy controls.
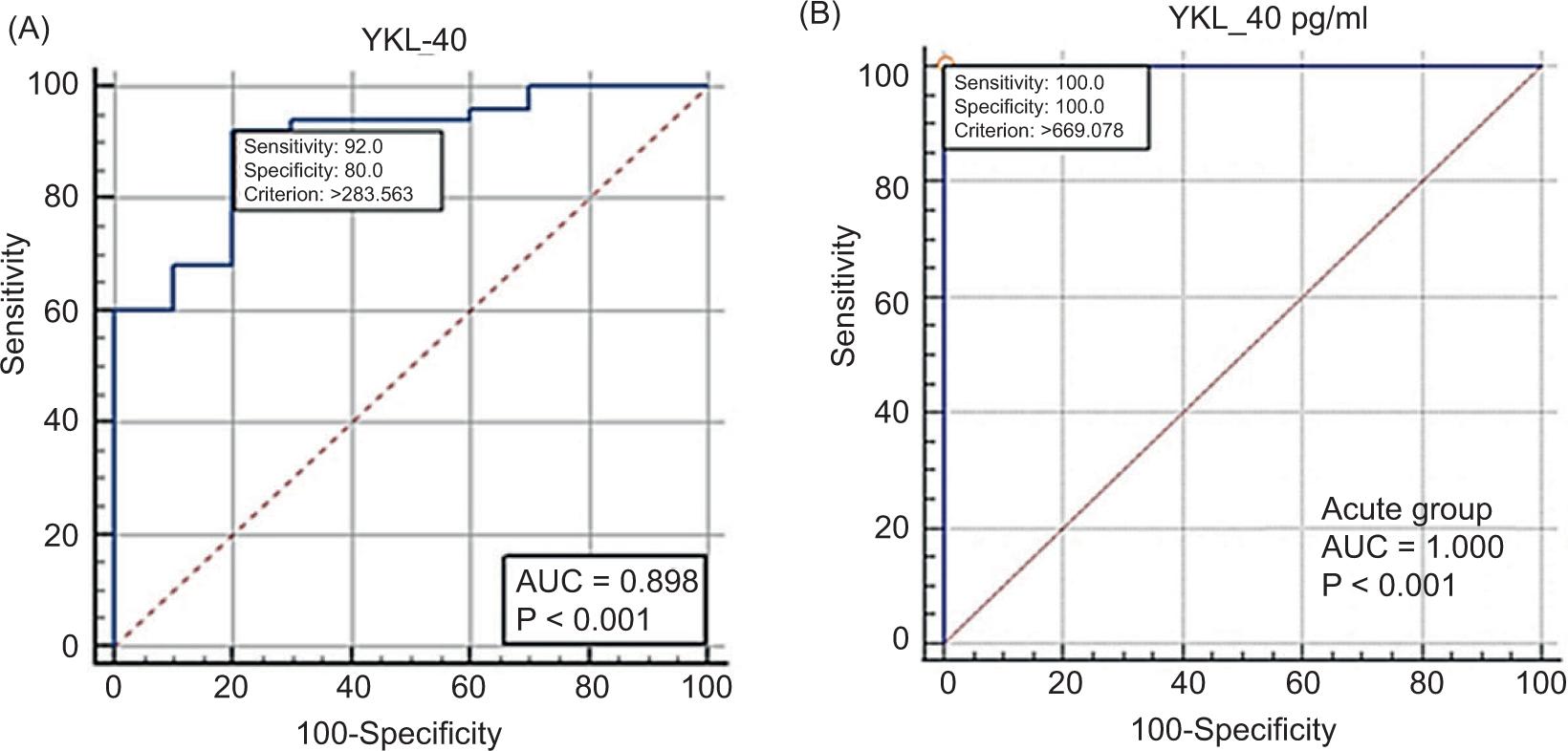
On the other hand, the comparison of the ROC curve and functional AUC between the chronic toxoplasmosis patients and healthy controls elucidated that YKL-40 was close to an excellent parameter, where the ROC curve showed AUC of YKL-40 as 0.898 and the cutoff value as >283.563. The sensitivity and specificity were 92 and 80, respectively; therefore, the YKL-40 can be considered a very highly significant biomarker (P < 0.0001) in the detection of the chronic toxoplasmosis (Table 2 and Figure 2b).
DISCUSSION
In this study, the first step performed after the collection of serum samples was a confirmatory investigation on the immunoglobulin status of the two basic types of anti-Toxoplasma antibodies, IgM and IgG, in the patients and control sera. This was to ensure the presence of the toxoplasmosis infection in the patients’ group and to ensure whether they had acute or chronic toxoplasmosis to distribute their samples into two groups for the later work. The levels of the specific anti-Toxoplasma IgM and IgG antibodies were adopted for this purpose. The statistical analysis of the recorded levels of both immunoglobulin types revealed that the levels of the IgM were higher in the patients previously diagnosed with acute infection, whereas the IgG levels were higher in those patients previously diagnosed with a chronic disease, a finding similar to that of the previous studies.45–50
Concerning the YKL-40, to our best knowledge, the relationship between the level of the YKL-40 and toxoplasmosis and between this protein and the phase of the disease (acute or chronic) has been studied for the first time globally in this study. Although there is no proven evidence that the YKL-40 is expressed at significant levels in toxoplasmosis to the extent that can be used in the detection of the disease, there is no doubt that an increased level of this protein may highly possibly be secreted during the infection, and that these levels may be proportional to the severity of the disease. This assumption is based on the previously observed data about parasite development and pathogenicity, which revealed that the parasite induces acute inflammatory responses in the infected tissues and encysts in the muscular and neural tissues. On the other hand, previous studies have documented the major role of YKL-40 in the inflammatory reactions, tissue injuries, remodeling, and repair,43,51,52 and polarization of Th2,40,53,54 which are all from the induced changes and mechanisms during the infection and the conflict against the parasite invasion. In addition, as variety of cells, including macrophages, neutrophils, SMC and FLC, are known to secrete the YKL-4040,41,55,56 and essentially engaged in fighting the parasite and inducing an immune response,57,58 it has been expected that excessive secretion of this protein may be associated with the immune response against the infection with the parasite.
Interestingly, the statistical analysis showed very highly significantly different levels of YKL-40 in toxoplasmic patients compared with the controls, and very highly significantly different levels were also observed between the two groups of patients with much higher levels in the acute infection group. Based on the pairwise comparisons between the pairs: Acute–Chronic, Acute–Control, and Chronic–Control, it can be said that YKL-40 has a sophisticated role in the differentiation between the infected and noninfected subjects on one hand and between the acute and chronic infections on the other.
Yet, the final evaluation was performed to estimate the sensitivity and specificity of the cutoff value of the YKL-40 at which the patients are considered infected with acute or chronic toxoplasmosis. The comparison of the ROC curve and the AUC between the acute toxoplasmic patients and healthy controls elucidated that YKL-40 is an excellent parameter. Accordingly, its sensitivity and specificity were 100. Therefore, the YKL-40 can be considered a very highly sensitive biomarker in the detection of acute toxoplasmosis. On the other hand, comparing the ROC curve and functional AUC between the chronic toxoplasmic patients and healthy controls elucidated that YKL-40 is a nearly excellent parameter. Accordingly, the sensitivity and specificity were 92 and 80, respectively. Therefore, the YKL-40 can also be considered a very highly sensitive biomarker in detecting chronic toxoplasmosis.
CONCLUSIONS
The high sensitivity and specificity of YKL-40 observed in this study classified the protein as a unique biomarker in the detection of toxoplasmosis. Additionally, the significantly different levels of YKL-40 between the groups of acute and chronic infections render the YKL-40 as a sophisticated biomarker that can vitally be used to detect the phase of the disease whether acute or chronic, besides its ability to detect the infection.