INTRODUCTION
Psoriasis (Ps) is a multisystemic chronic inflammatory disease caused by a genetic factor that affects 2%–3% of the global population.2 Psoriasis vulgaris accounts for approximately 85% to 90% of all psoriasis patients.3,4
The pathophysiology of psoriasis is complicated, and many processes involved are yet to be identified. The inflammatory response is influenced by a complicated interplay between genetic susceptibility and environmental factors.1 The condition has the potential to drastically damage the assaulted patient’s physical and psychological function, as well as have a direct negative impact on their quality of life.5,6 Several techniques have been used to assess the severity of psoriasis and its influence on quality of life.
The Psoriasis Area and Severity Index (PASI) is a clinical tool for assessing psoriasis severity. It combines severity parameters (erythema, induration, and desquamation, and the percentage of affected area).7,8
The issue with the Dermatology Life Quality Index (DLQI) is that it is used to assess quality of a good life in six sections. These are also influenced by skin disease namely, symptoms and sentiments, daily activities, leisure, job, personal relationships, and treatment discomfort.9 Both PASI and DLQI are among the most quoted and frequently used psoriasis evaluation methods because of their high level of reliability, applicability, and reproducibility.
Psoriatic patients with a PASI or DLQI score <10 are said to have mild psoriasis, whereas those with scores >10 are said to have moderate to severe psoriasis.10 Psoriasis is a chronic, hyperproliferative inflammatory disease in which oxidative stress may play a pathogenetic role.11,12 In chronic inflammatory disease,13 disturbances in the pro-oxidant and antioxidant balance favor the first, resulting in excessive formation of reactive oxygen species (ROS), such as superoxide (•O2) and hydrogen peroxide (H2O2).14 Excessive ROS causes a variety of biological changes, including DNA alterations, increased lipid peroxidation, and the generation of inflammatory cytokines.15,11 On the other hand, there is a lack of antioxidants that plays a role in the pathogenesis of psoriasis, as evidenced by a decrease in the antioxidants molecules essential for neutralizing the free radical load.16 As a result, an antioxidant molecule may be a viable therapeutic alternative.
Coenzyme Q10 (CoQ10) ubiquinone, is a compound that is naturally present in the human body as a component of the electron transport chain in mitochondria (the major source of ROS).17 Several studies have pointed out that CoQ10 can damage free radicals or even totally prevent the damages that may have resulted. Consequently, they improve energy, and build up the immune system.18
Nevertheless, other factors have been noted that take part in the pathogenesis process of psoriasis: dermal, systemic expression of proinflammatory cytokines, especially interleukins (ILs), Tumor Necrosis Factor α (TNFα) and Interferon γ (IFNγ), as well as a complex molecular interactions between epidermal keratinocyte, mononuclear leukocytes, neutrophil and activated T-cells.15 Adalimumab (Amgevita®) and etanercept (Enbrel®) are anti-TNF agents that either bind to TNF, inhibiting receptor binding, or block TNF receptor activation. Advances in biotechnology have the potential to offer greater safety by developing drugs that interfere with specific targets in the pathogenesis of psoriasis. Both medications are currently accessible to treat psoriasis.19 The goal of this 12-week clinical experiment was to compare the percentage improvement in quality of life and psoriasis clinical severity in Iraqi psoriatic patients with persistent plaque psoriasis treated before and after adjuvant therapy with the biological agent Adalimumab in combination with CoQ10.
PATIENTS AND METHODS
During the months of January to November 2021, a prospective double-blinded clinical trial on 38 patients with persistent plaque psoriasis and a clinical indication for biological treatment was conducted in the Department of Dermatology at the Merjan Teaching Hospital in Babylon, Iraq. Only 24 patients successfully completed the study period out of the 38 who volunteered to participate in the experiment and signed a consent form approved by the local research ethics council. The participants with age ranging from 17 to 72 years consisting 9 women (37.5%) and 15 men (62.5%) were divided into two groups: a biological medicine (adalimumab) and a placebo (cornstarch) were given to Group A (n = 11), whereas Group B (n = 13) received 100 mg CoQ10 adjuvant therapy in addition to the biological medication already provided. The PASI score was used to determine the severity of the disease in all of the patients by the same dermatologist. In addition, the patients were asked to complete a standardized quality of life questionnaire during a single appointment (DLQI). There were 10 questions in this tool, which were separated into six categories (symptoms and feelings, daily activities, leisure, job and school, personal connections, and psoriasis treatment discomfort). Each item is rated on a four-point scale, with alternatives such as “not at all”, “a little”, “a lot”, and “very much”. The total score9,20,21 is derived by combining the item scores together.22
The biological treatment consisted of twice-monthly subcutaneous administration of 40 mg adalimumab (Amgevita®), a typical biological medication. After day 90 of treatment, patients were reviewed and clinically evaluated by the same physician. Regardless of their present medical status, they all completed the same standardized questionnaire.
Inclusion Criteria
Patients with psoriasis vulgaris who have a PASI score >10 were considered eligible.
Exclusion Criteria
Those with other chronic illnesses, such as diabetes mellitus, liver and renal problems, pregnant or breastfeeding participants, and those under the age of 17 were eliminated.
Statistical analysis
The statistical software SPSS® version 25 for Windows was used to perform the statistical analysis. The data is presented in the form of mean values and standard deviation (SD). The Shapiro–Wilk test was employed to ensure that the dependent variables’ distributions were normal. The Wilcoxon test showed the differences in the means of two groups. Statistical significance was defined as Asymptotic Significance (two-tailed) at 0.05. For non-parametric data, Spearman’s correlation and Spearman’s correlation coefficient were used for correlation analysis.
RESULTS
Table 1 shows the demographic profile of patients who completed all questions satisfactorily at baseline and three months after the completion of treatment (n = 24). With an age range of 17 to 72 years, participant group A had a mean SD age of 44.13 ± 12.72 for males and 44.33 ± 20.59 for females, whereas group B had a mean SD age of 44.71 ± 8.19 for males and 30.17 ± 12.64 for females.
TABLE 1. Descriptive statistic for the study groups.
| Group | Gender | N | Age (mean ± SD) |
|---|---|---|---|
| A | Male | 8 | 44.13 ± 12.72 |
| Female | 3 | 44.33 ± 20.59 | |
| B | Male | 7 | 44.71 ± 8.19 |
| Female | 6 | 30.17 ± 12.64 |
Table 2 shows mean PASI and DLQI scores, as well as mean percentage changes, for all patients enrolled in this study before and after treatment. The mean SD baseline PASI and DLQI scores were 20.88 ± 7.15 and 12.50 ± 4.72 for PASI and DLQI score, respectively. After 12 weeks of biological and adjuvant CoQ10 treatment, significant reductions in the two scores were reported to be (p= 0.05). The mean percentage increase change using the PASI score was 67.48 ± 22.25, whereas the DLQI score improved by 56.13 ± 20.15, indicating a significant positive connection between the two scores (p value = 0.05).
TABLE 2. The Mean±SD PASI and DLQI Scores and Mean ± SD percentage changes for all patients enrolled in this study before and after treatment.
| Assessment tool | N | Mean ± SD before treatment |
Mean ± SD after treatment |
Asymptotic significance (2-tailed) | Mean ± SD Percentage improvement change |
Asymp. Sig. (2-tailed) |
|---|---|---|---|---|---|---|
| PASI | 24 | 20.88 ± 7.15 | 7.08 ± 6.68* | .000 | 67.48 ± 22.25a | .000 |
| DLQI | 24 | 12.5 ± 4.72 | 5.50 ± 3.20* | 56.13 ± 20.15 |
*Statistically significant level before and after treatment. Asymptotic Significance (2-tailed, p < 0.05).
aStatistically significant level between PASI and DLQI percentage improvement changes p < 0.05).
Table 3 shows the mean ± SD PASI and DLQI scores and percentage changes for each group before and after therapy, revealing that both PASI and DLQI scores were significantly reduced after 12 weeks treatment (7.08 ± 6.68, 5.50 ± 3.20, respectively, P=0.05). After 12 weeks of treatment, the percentage improvement changes in PASI and DLQI for group A (getting biological therapy with placebo) were (45.71 ± 12.13, 36.67 ± 8.13), respectively. The percentage improvement for PASI and DLQI were (85.91 ± 4.89, 72.60 ± 9.07) when CoQ10 was used as adjuvant therapy in group B.
TABLE 3 The Mean ± SD PASI and DLQI Scores and Mean ± SD Percentage Changes for Each Group before and after Treatment.
| Assessment tool | N | Group A | N | Group B | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± SD before treatment |
Mean ± SD after treatment |
Mean ± SD percentage improvement change |
Mean ± SD before treatment |
Mean ± SD after treatment |
Mean ± SD percentage improvement change |
Asymp. Sig. (2-tailed) | |||
| PASI | 11 | 22.15 ± 8.40 | 12.37 ± 6.69 | 45.71 ± 12.13 | 13 | 19.80 ± 6.04 | 2.61 ± 0.92 | 85.91 ± 4.89a | 0.00 |
| DLQI | 11 | 12.73 ± 4.79 | 7.91 ± 2.66 | 36.67 ± 8.13 | 13 | 12.31 ± 4.84 | 3.46 ± 1.98 | 72.59 ± 9.07 | 0.00 |
aStatistically significant level between PASI and DLQI percentage improvement changes p < (0.05).
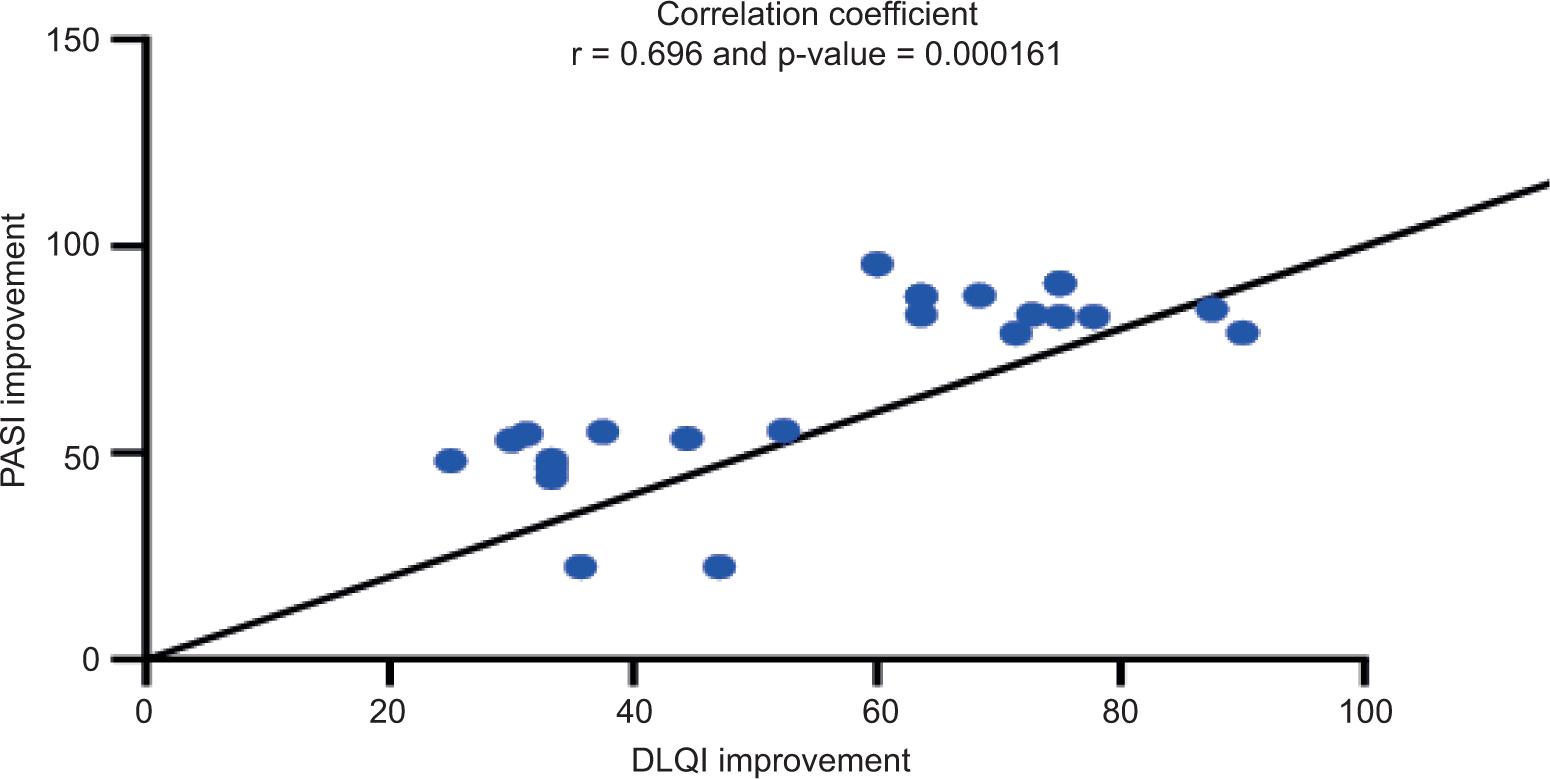
Table 4 shows the association between PASI and DLQI scores before and after treatment, revealing a highly significant positive correlation (r = 0.702, P = 0.000132) between PASI and DLQI scores after 12 weeks of therapy. Before starting therapy, there was no discernible link between PASI and DLQI levels (r = 0.13, P = 0.545). Changes in percentage improvement for both PASI and DLQI showed a highly significant association (r = 0.696, p = 0.000161). Figures 2 and Table 3 shows the Spearman’s correlation coefficients for the correlation between PASI and DLQI scores and percentage improvement changes after treatment time, respectively.
TABLE 4. The Correlation between both PASI and DLQI scores and percentage improvement changes before and after treatment.
| Correlated variables | r | p-value |
|---|---|---|
| PASI with DLQI Before treatment | 0.130 | 0.545 |
| PASI with DLQI After treatment | 0.702** | 0.000132 |
| PASI with DLQI Improvement | 0.696** | 0.000161 |
**Correlation is highly significant (Spearman’s correlation coefficient) at the 0.01 level (two-tailed).
FIGURE 1. The Difference in Mean Percentage Improvement Changes of PASI and DLQI Scores Between group A and group B.
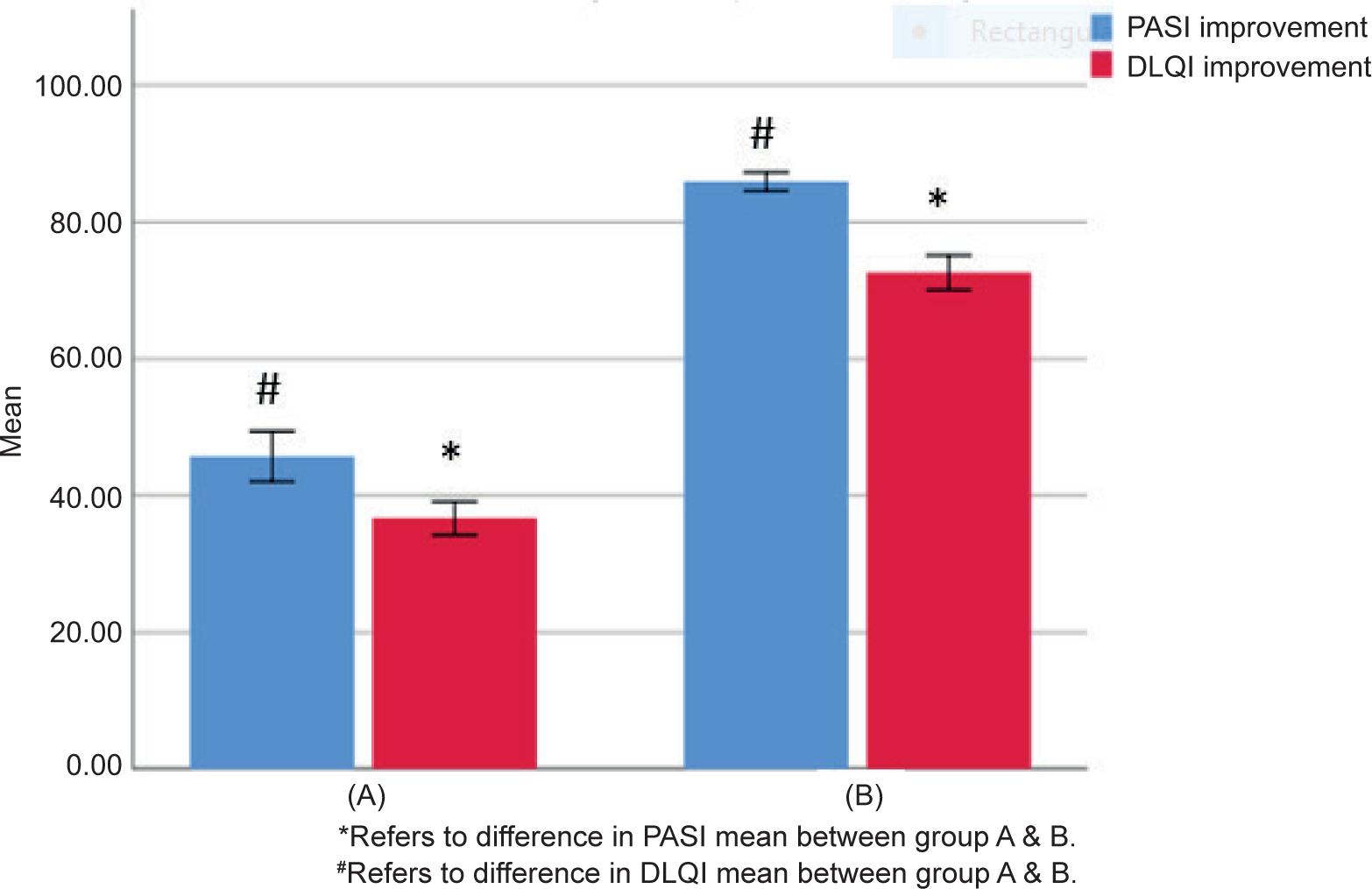
FIGURE 2. Correlation between PASI and DLQI Scores after 12 Weeks Treatment.
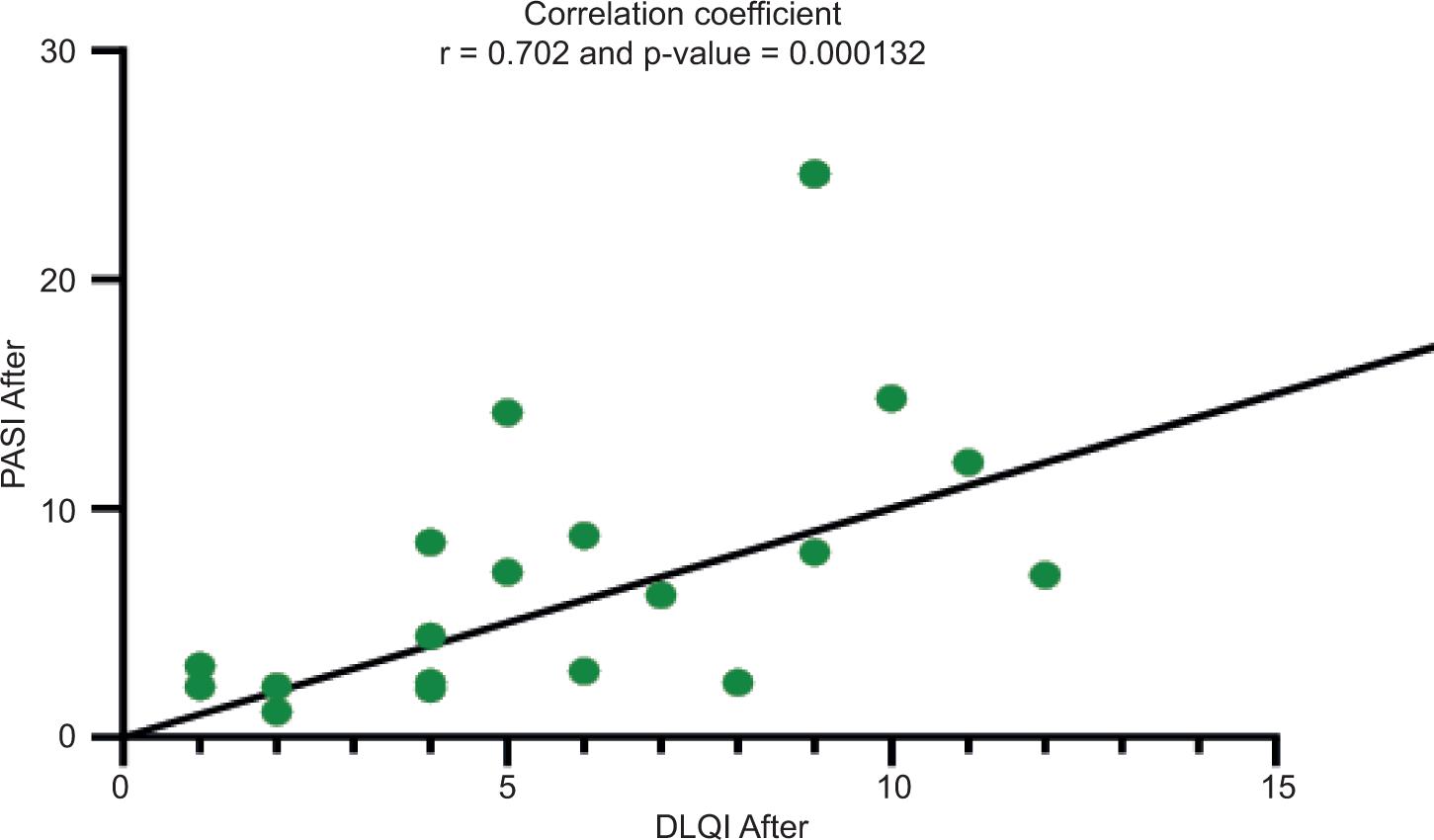
FIGURE 3. Correlation between PASI and DLQI Percentage Improvement Changes after 12 Weeks Treatment.
DISCUSSION
The PASI and DLQI scores were used to examine the influence of changes in psoriasis severity on quality of life in patients with psoriasis at baseline and 3 months after treatment with biological therapy and an adjuvant CoQ10. Psoriasis has a detrimental impact on quality of life, and numerous research have shown how psoriasis severity affects quality of life.23–25 Although Psoriasis is a chronic, incurable condition, clinicians’ primary goal is to enhance patients’ quality of life, particularly those with severe psoriasis.
Dermatologists use the PASI score to assess psoriasis to obtain more objective clinical measures, but there are always limitations, such as the score calculation being complex, the affected area being subjective estimates, and most importantly, the scoring not taking into account the impact on the patient’s quality of life.26
The PASI and DLQI scores were calculated in this investigation. Before treatment with the biological therapy and an adjuvant antioxidant, there was no correlation between PASI and DLQI in both groups at baseline; however, after treatment with the biological therapy and an adjuvant antioxidant, both PASI and DLQI showed strong correlation and significant improvement in both scores when compared to baseline scores.
Regardless of treatment type, the percentage improvement change for PASI score was significantly higher than that for DLQI score at the end of treatment. This could be explained by the fact that DLQI as a measure of assessment is often influenced by social and cultural factors.9
The severity of the disease and the long-term clinical course history of psoriasis symptoms, on the other hand, have a negative impact on DLQI due to psychological involvement in such patients, which further hampered their treatment compliance.
The findings of this investigation corroborated those of several earlier studies, which found a strong link between disease severity and quality of life.9,27,28,37,38 Other research, such as.6,29 have found no link between PASI and DLQI.30-33
Clinical investigations on psoriasis patients have showed that utilizing CoQ10 as an adjuvant therapy improves severity indicators and quality of life significantly.1,34,35
In a study by Kharaeva et al.,35,36 58 psoriasis patients were treated with CoQ10 for one month and showed normalization of all oxidative stress markers produced by the blood and skin compartments, as well as improvements in skin structure and function. Biological therapies, alone or in combination with coenzyme Q10 supplements, may play a significant role in boosting the immune system and physical performance, the reason for this is that tissues and cells involved in immune function are highly energy-dependent, which is essential in powering the body’s energy production ATP cycle, and thus require an adequate supply of CoQ10 for optimal performance.
We investigated the effect of CoQ10 on the therapeutic value of biological therapy in psoriatic Iraqi patients in this study. When CoQ10 was combined with a biological medicine, it resulted in a large decrease in PASI score as well as a significant increase in PASI and DLQI percentage improvement changes.
The findings of the above-mentioned studies clearly support the findings of this study; thus, it can be concluded based on the obtained results that administering CoQ10 (100 mg/day) as adjuvant therapy to psoriatic patients for 12 weeks had beneficial effects on the correlation between the PASI and DLQI after treatment with biological drugs.
ETHICAL APPROVAL
The manuscript is written in original and all the data, results pertaining to this manuscript are original according to the research performed. The authors followed academic integrity and have not copied any content/results from another source.