INTRODUCTION
Breast cancer is becoming a second major source of illness and death around the world. Furthermore, for researchers, the rising rate of breast cancer remains a key source of concern. Increased public awareness leads to more recurrent medical exams and diagnostic imaging, resulting in earlier diagnoses and therefore improved prognosis.1–4 Magnetic resonance imaging (MRI), in addition to mammography and ultrasound, is extremely useful in the detection of breast cancer due to its greater sensitivity and specificity.5–7 The use of MRI in various areas of breast cancer diagnosis and therapy has been made possible by significant advancements in MRI techniques, that helped in precise cancer diagnosis and anatomic identification.8–10
The sensitivity of dynamic contrast-enhanced MRI (DCE-MRI) in detecting breast cancer is rather high, with a range of 88%–100% for invasive breast cancers (Figure 1)7,11,12 The observed specificity of DCE-MRI, on the other hand, has been widely disparate from 37% to 97%. The specificity of DCE-MRI varies depending on the lesion criteria utilized to differentiate between benign and malignant breast lesions.5 Lesions morphology and enhancement kinetics are two widely utilized lesion criteria in identifying breast lesions by DCE-MRI.13,14
FIGURE 1. DCE-MRI and time-signal intensity curve: left breast lesion.
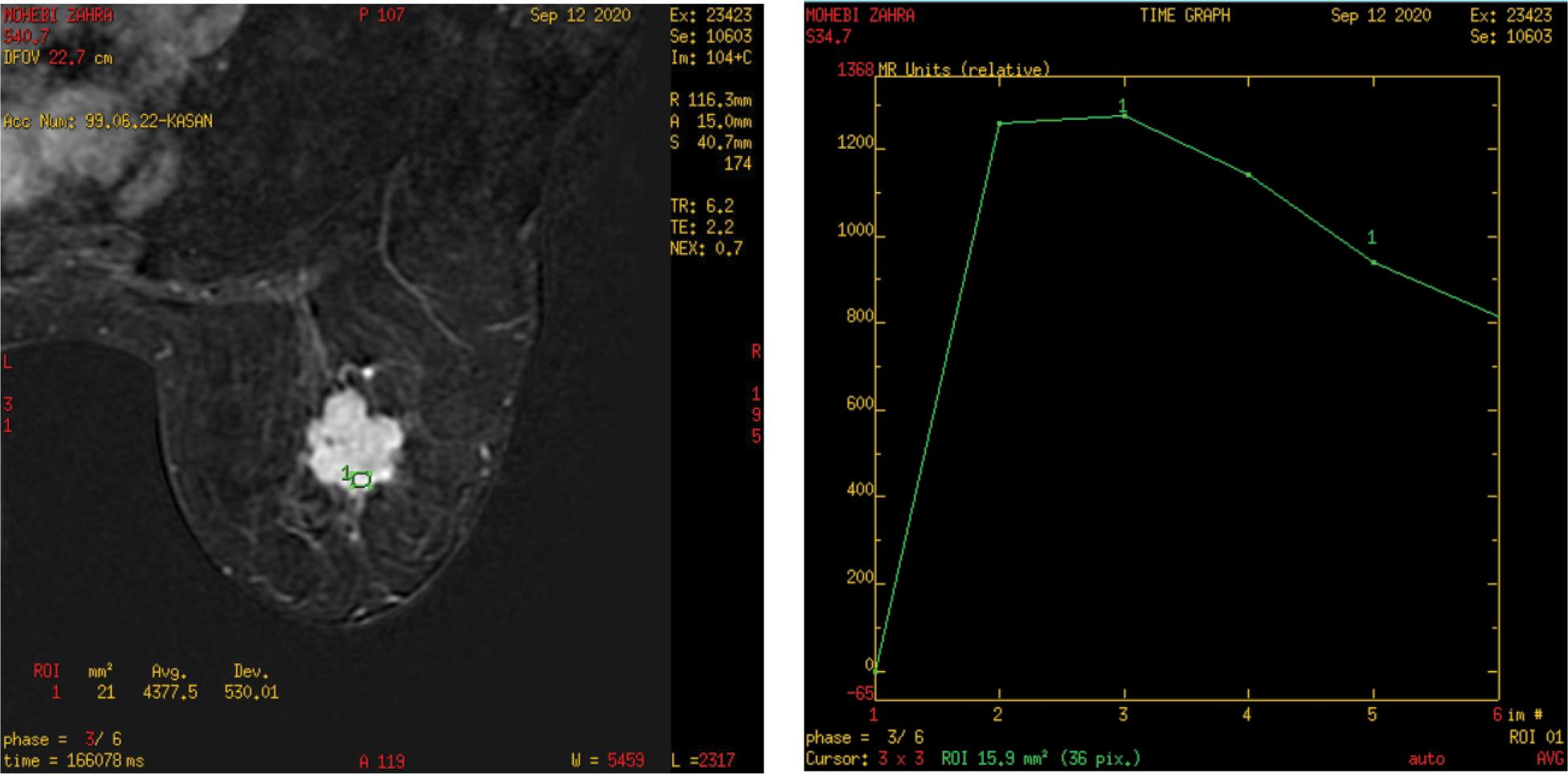
The morphological assessment of breast lesions is done by assessing their form, margins, enhancement features, enhancement distribution, and internal enhancement pattern, according to the breast imaging reporting & data system (BI-RAD) MRI lexicon. The initial and post-initial enhancement of the breast lesion is detected during kinetic assessment.15–17
The goal of this study was to see how well DCE-MRI may separate benign and malignant breast tumors.
PATIENTS AND METHODS
This prospective study was conducted in a private medical imaging center between October 2020 and June 2021. The study included 32 women (ages 25 to 75; mean age 46.6 years) who had 32 suspicious breast lesions identified via physical examination, mammography, and ultrasonography.
All of the patients had a detailed history taken with a general and local examination. All patients had conventional MRI and DCE-MRI examinations. The findings of breast MRI were compared to the histopathology results, which were utilized as a gold standard. Patients who agreed to participate in the study gave their informed consent, and the ethics committee approved the study.
A 3-T magnetic resonance (GE) equipment was used to evaluate all of the patients. A specialized breast coil was used to examine all patients in the prone position. The examination included image acquisition followed by image post-processing.
The protocol suggested for breast exam was:
-
T2-weighted fast spin-echo sequence
-
T1-weighted non-fat-suppressed sequence
-
DW sequence
-
3-dimensional T1-weighted fat-suppressed DCE sequence
Imaging parameters of DCE-MRI were as follows:
-
repetition time = 4.1
-
echo time = 2.1
-
field of view = 28 cm
-
nex = 0.71
-
matrix = 300 × 300
-
slice thickness = 2 mm
-
gap = 0
The images obtained with 6 post-contrast acquisitions were centered at 40, 120, 200, 280, 360, and 440s.
RESULT
For their suspicious breast lesions, all 32 patients in this research have taken DCE-MRI. Their index lesion was also subjected to a histopathologic reference standard test. In 14 patients (43.75%), histopathologic examination revealed benign lesions, while in 18 individuals, malignant lesions were discovered (56.25&). Types of histopathology of 14 benign lesions are listed in Table 1 as follows: 5 lesions (35.71%) were fibroadenomas, 3 lesions (21.42%) were fibrocystic changes (FCC), 2 lesions (14.28%) were mastitis, 2 lesions (14.28%) were fat necrosis, 1 lesion (7.14%) was a postoperative scar, and 1 lesion (7.14%) was postoperative seroma.
TABLE 1. 14 benign breast lesions with histopathological diagnoses.
| Histopathological type | No. | % |
|---|---|---|
| Fibroadenoma | 5 | 35.71 |
| Fibrocystic change | 3 | 21.42 |
| Mastitis | 2 | 14.28 |
| Fat necrosis | 2 | 14.28 |
| Postoperative scar | 1 | 7.14 |
| Postoperative seroma | 1 | 7.14 |
| Total | 14 |
The histopathologic types of 18 malignant tumors are listed in Table 2: 6 lesions (33.33%) had invasive duct carcinoma (IDC), 4 lesions (22.22%) had invasive lobular carcinoma (ILC), 3 lesions (16.66%) had mucinous carcinoma, 3 lesions (16.66%) had medullary carcinoma, and 2 lesions (11.11%) had ductal carcinoma in situ (DCI).
TABLE 2. 18 malignant breast lesions with histopathological diagnoses.
| Histopathological type | No. | % |
|---|---|---|
| Invasive duct carcinoma (IDC) | 6 | 33.33 |
| Invasive lobular carcinoma (ILC) | 4 | 22.22 |
| Mucinous carcinoma | 3 | 16.66 |
| Medullary carcinoma | 3 | 16.66 |
| Ductal carcinoma in situ (DCI) | 2 | 11.11 |
| Total | 18 |
The average dimension of benign lesions was 2.7 cm, with a range of 1–7.5 cm, whereas malignant lesions were 2.9 cm, with a range of 2–6.8 cm (Table 3).
TABLE 3. A comparison of histopathological data in terms of lesion size.
| Size (cm) | Benign | Malignant | p |
|---|---|---|---|
| Average | 2.7 | 2.9 | 0.46 |
| Range | 1–7.5 | 2–6.8 |
p: probability; Mann–Whitney U-test used.
There were four rounded lesions, all of which were benign, depending on the form of lesions. There were seven ovoid lesions in all, which were benign. There were 10 lobulated lesions, four of which were benign and six were malignant; and 11 irregular lesions, four of which were benign, and seven were malignant. There were eight smooth margin lesions, all of which were benign; 14 irregular margin lesions, four of which were benign and 10 were malignant; and 10 hypothesized margin lesions, three of which were benign and seven were malignant (Table 4).
TABLE 4. The morphologic features of breast lesions (in terms of form and margin) in connection to histological results.
| Benign | Malignant | p | |||
|---|---|---|---|---|---|
| Shape | Rounded | No | 4 | 0 | <0.0001 |
| % | 12.5% | 0.0% | |||
| Ovoid | No | 7 | 0 | ||
| % | 21.87% | 0.0% | |||
| Lobulated | No | 4 | 6 | ||
| % | 12.5% | 18.75% | |||
| Irregular | No | 5 | 6 | ||
| % | 15.62% | 18.75% | |||
| Margin | Smooth | No | 8 | 0 | <0.0001 |
| % | 25% | 0.0% | |||
| Irregular | No | 4 | 10 | ||
| % | 12.5% | 31.25% | |||
| Speculated | No | 3 | 7 | ||
| % | 9.37% | 21.87% |
p: probability; The Mann–Whitney U-test was employed.
Based on the contrast enhancement pattern of the tumors, homogenous enhancement was noted in nine tumors: six tumors were benign and three were malignant; heterogeneous enhancement was noted in 13 tumors: four tumors were benign and nine were malignant; rim enhancement was noted in seven tumors: three tumors were benign and four were malignant; and non-mass enhancement was noted in three tumors: one tumor was benign and two tumors were malignant. The wash-in rate was slow (50%) in five tumors, all of which were benign; moderate wash-in rate (50–80%) in 12 tumors, all of which were benign. Eight tumors were benign, whereas four were malignant; and 15 tumors had a high wash-in rate >80%, including one benign lesion and 14 malignant lesions. A persistent curve was seen in 12 tumors based on the form of the dynamic curve (time and signal intensity curve). There were 10 benign tumors and two malignant tumors; type II (plateau curve) was found in eight of the tumors: three tumors were benign, five tumors were malignant, and 12 tumors had type III (washout curve): one tumor was benign, but the other 11 were cancerous (Table 5).
TABLE 5. The enhancement pattern and enhancement kinetics (with regard to wash-in rate and shape of time/signal intensity curve) of breast lesions with histopathological results.
| Groups | p | |||||
|---|---|---|---|---|---|---|
| Benign | Malignant | |||||
| No. | % | No. | % | |||
| Enhancement pattern | Homogenous enhancement | 6 | 42.85 | 3 | 16.66 | <0.0001 |
| Heterogeneous enhancement | 4 | 28.57 | 9 | 50 | ||
| Rim enhancement | 3 | 21.42 | 4 | 22.22 | ||
| Non-mass enhancement | 1 | 7.14 | 2 | 11.11 | ||
| Wash in rate | Slow enhancement (<50%) | 5 | 35.71 | 0 | 0.0 | <0.0001 |
| Intermediate enhancement (50–80%) | 8 | 57.14 | 4 | 22.22 | ||
| Strong enhancement (>80%) | 1 | 7.14 | 14 | 77.77 | ||
| Shape of time/SI curve | Persistent type I | 11 | 78.57 | 1 | 5.55 | <0.0001 |
| Plateau type II | 2 | 14.28 | 6 | 33.33 | ||
| Washout type III | 1 | 7.14 | 11 | 61.11 | ||
p: probability; Mann–Whitney U-test employed.
DISCUSSION
Breast lesions may be detected with excellent accuracy using DCE-MRI. DCE-MRI has additional precision than mammography or ultrasonography for determining the extent of illness in patients with a recent cancer diagnosis but limited capacity to distinguish between benign and malignant lesions in individuals with a recent cancer diagnosis.
The study included 32 women (ages 25 to 75; mean age 46.6 years) who had 32 suspicious breast tumors identified using physical check-ups, mammography, and ultrasonography.
These 32 tumors in the study were divided into 14 benign tumors (43.75%) and 18 malignant (56.25%) tumors, according to the histopathological analysis.
In this study, all mass lesions were done which revealed 32 enhanced lesions. The homogenous enhancement lesions were nine: six lesions were benign and three lesions were malignant. The heterogeneous enhancement lesions were 13: four lesions were benign and nine were malignant. In rim enhancement, there were seven lesions: three lesions were benign and four were malignant. The non-mass lesions in the present study were three, one of them was benign and the other was malignant. The heterogeneous enhancement was shown to be suggestive of malignant lesions, whereas homogeneous enhancement is more likely to occur in benign lesions. While there are no particular criteria for the enhanced pattern in non-mass lesions, this supports the findings of Tozaki et al.18,19
We observed eight tumors with smooth margins (well defined) and all were benign while the other tumors with irregular and speculated margins were mostly malignant. This is similar to Macura et al.’s20,21 study. According to them, the margin description of a focal mass is the most predictive characteristic of breast MRI interpretation, and hypothesized margins are more worrisome for cancer. The time-signal intensity curve of DCE-MRI revealed 12 lesions that showed a progressive raising curve (type I curve), 11 lesions were benign and one lesion was malignant by histopathology diagnoses. Eight lesions showed a plateau curve (type II curve), in which two lesions were benign and six were malignant. Twelve lesions showed rapid washout (type III curve), 11 of them were proved by histopathology as malignant. This is congruent with numerous studies like Schnall et al.,22,23 which demonstrated the relevance of the curve form in distinguishing between malignant and benign tumors. The application of time-signal intensity curves resulted in substantially better discrimination between benign and malignant tumors. Persistent curves are linked with benign lesions, but type III curves are more suggestive of malignant. Plateau curves can indicate whether a lesion is cancerous or benign.24–30
STUDY LIMITATION
The main limitation of our study was the pandemic of coronavirus which caused a decrease in number of participants.