INTRODUCTION
Crohn’s disease is a chronic inflammatory disorder that may involve any part of the alimentary tract from mouth to anus, but with a propensity for the distal small intestine and proximal large bowel. Inflammation in Crohn’s disease often is discontinuous along the longitudinal axis of the intestine and can involve all layers from mucosa to serosa. The presentation of Crohn’s disease may be subtle and varies considerably according to factors like the location of disease, the intensity of inflammation, and the presence of specific intestinal and extraintestinal complications.1 Crohn’s disease is most commonly present in the terminal ileum, with at least 60% of patients having at least some ileal involvement.2 Of these, about half have isolated small bowel involvement while the remainder also has additional colonic involvement (usually affecting the right side greater than the left). Twenty percent of patients with Crohn’s disease have isolated colonic involvement, which can sometimes present a challenge when trying to distinguish Crohn’s disease from ulcerative colitis. About 5% of patients have upper-tract involvement, which can affect the esophagus, stomach, duodenum, and/or jejunum in addition to the distal small bowel.3 Perianal Crohn’s disease may occur in isolation or, more commonly, in association with disease elsewhere in the GI tract, usually a distal luminal site.4 Crohn’s disease is characterized by periods of clinical remission alternating with periods of recurrence. Persistent inflammation is believed to lead to progressive bowel damage that, over time, will manifest in the development of strictures, fistulae, and abscesses.5–7 These complications frequently lead to loss of function and need for surgical resection, which, in turn, can lead to disability.8,9 As in other chronic inflammatory diseases, such as rheumatoid arthritis, the treatment paradigm in Crohn’s disease is currently shifting from mere symptom control toward the reduction of long-term disease sequelae. The definition of remission is evolving and has moved beyond the Crohn’s disease activity index and other subjective disease activity indices to include mucosal and histologic healing, which are more closely associated with improved long-term goals and preventing bowel damage.10 There is increasing evidence that clinical remission of symptoms does not reflect underlying healing of inflammation (subclinical inflammation often persists) or reduced risks of future penetrating and fibrostenosing complications that might require surgery. In contrast to symptom improvement, mucosal healing has been associated with a reduction in hospitalization, need for future steroids and surgery,11 as well as a greater likelihood of maintaining remission off biological therapy among patients treated with combination therapy of both a biological and an immunomodulator.12 It also theoretically lower the likelihood of colon cancer among patients with longstanding colonic inflammation.13 Thus, the concept of treating toward the goal of mucosal healing has inherent appeal, although the most meaningful and practical criteria for mucosal healing (endoscopic or histological) remain to be clarified, given that mucosal inflammatory activity is itself a surrogate for future fibrosis/stenosis and irreversible bowel damage.14
Several clinical (e.g., the Crohn’s disease activity index and the simpler Harvey–Bradshaw index) and endoscopic (e.g., the simple endoscopic score for Crohn’s disease and the Crohn’s disease endoscopic index of severity) indices are currently used both in clinical trials and clinical practice to measure the severity of Crohn’s disease and its impact on quality of life. These indices only measure disease activity at a specific time point. The recently published Lémann index (Crohn’s disease digestive tract damage score) is the first tool that aims to measure the cumulative structural bowel damage, including strictures, penetrating lesions (abscesses and fistulae), and surgical resection.15 This evaluation includes damage location, extent, severity, progression, and reversibility (measured by imaging techniques and the need for resection). There are numerous future potential applications for this index, including the assessment of disease progression and patient’s heterogeneity, the identification of parameters at diagnosis associated with a high risk of rapid progression, and the impact of therapy strategies on long-term outcomes.15,16
PATIENTS AND METHODS
This is a descriptive, single-center study conducted in a gastroenterology and hepatology teaching hospital (Baghdad) and enrolled 30 patients (19 male and 11 female) diagnosed with CD from 2013 to 2015. All of the 30 patients underwent complete physical examination, abdominopelvic CT scan (oral and intravenous contrast), upper endoscopy, and colonoscopy upon diagnosis. Exclusion criteria were female patients with known or suspected pregnancy, renal impairment, and lack of imaging study prior to starting treatment. For the analysis and creation of the LI, the digestive tract was divided into four organs: upper digestive tract, small bowel, colon/rectum, and anus. Each organ was further divided into segments: three segments for the upper digestive tract (esophagus, stomach, and duodenum), six for the colon/rectum (cecum, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum), and one for the anus. For small bowel, each lesion within 20-cm length was considered to represent one small bowel segment, and the number of segments was capped at 20 (Figure 1). Surgical procedures, predefined strictures and/or penetrating lesions were graded on a 3-point severity scale. A segmental score ranging from 0 no lesion) to 10 complete resection of the segment) is given, taking into account the presence and severity of lesions (stricturing or penetrating) (Table 1, Figure 2). For each organ, a cumulative damage evaluation was then calculated as the sum of segmental damage evaluations provided plus the damage attributed to resected segments in case of previous total resection (10 for each segment), taking into account the individual relative weights attributed by the investigator to each segment within the organ. Finally, a global score is calculated, taking into account the four organ damage scores (see Tables 2 and 3 and Figure 3).17
Figure 1. Assessment of digestive damage using the Lémann score: segmentation of the digestive tract. To calculate the score, the digestive tract is divided into four organs (upper digestive tract, small bowel, colon and rectum, and anus); then each organ is divided into segments, which are individually scored for damage on an ordinal scale ranging from 0 (normal) to 3 (maximal).
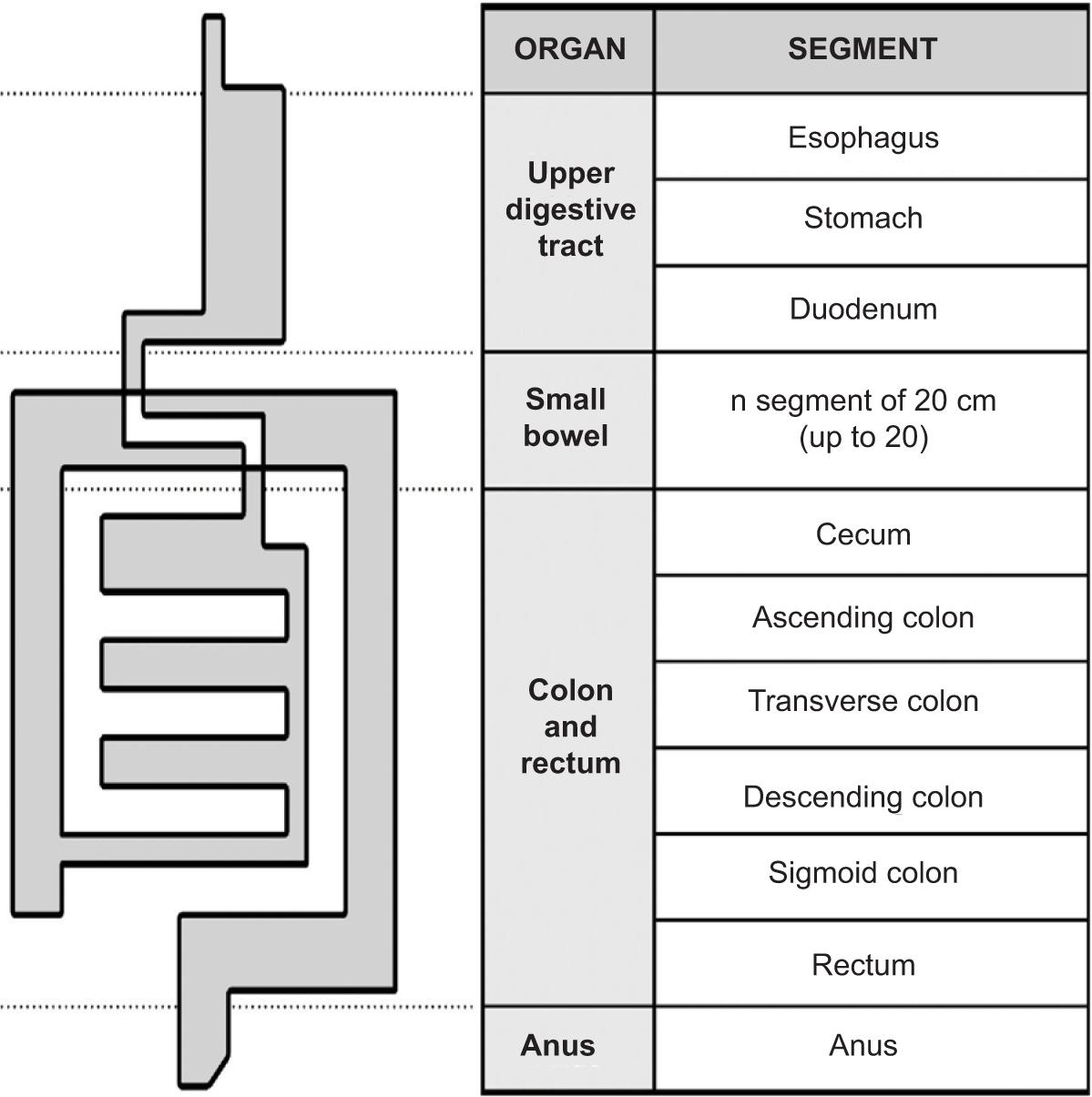
Figure 2. CT scan of the patient showing stricturing and penetrating lesions.
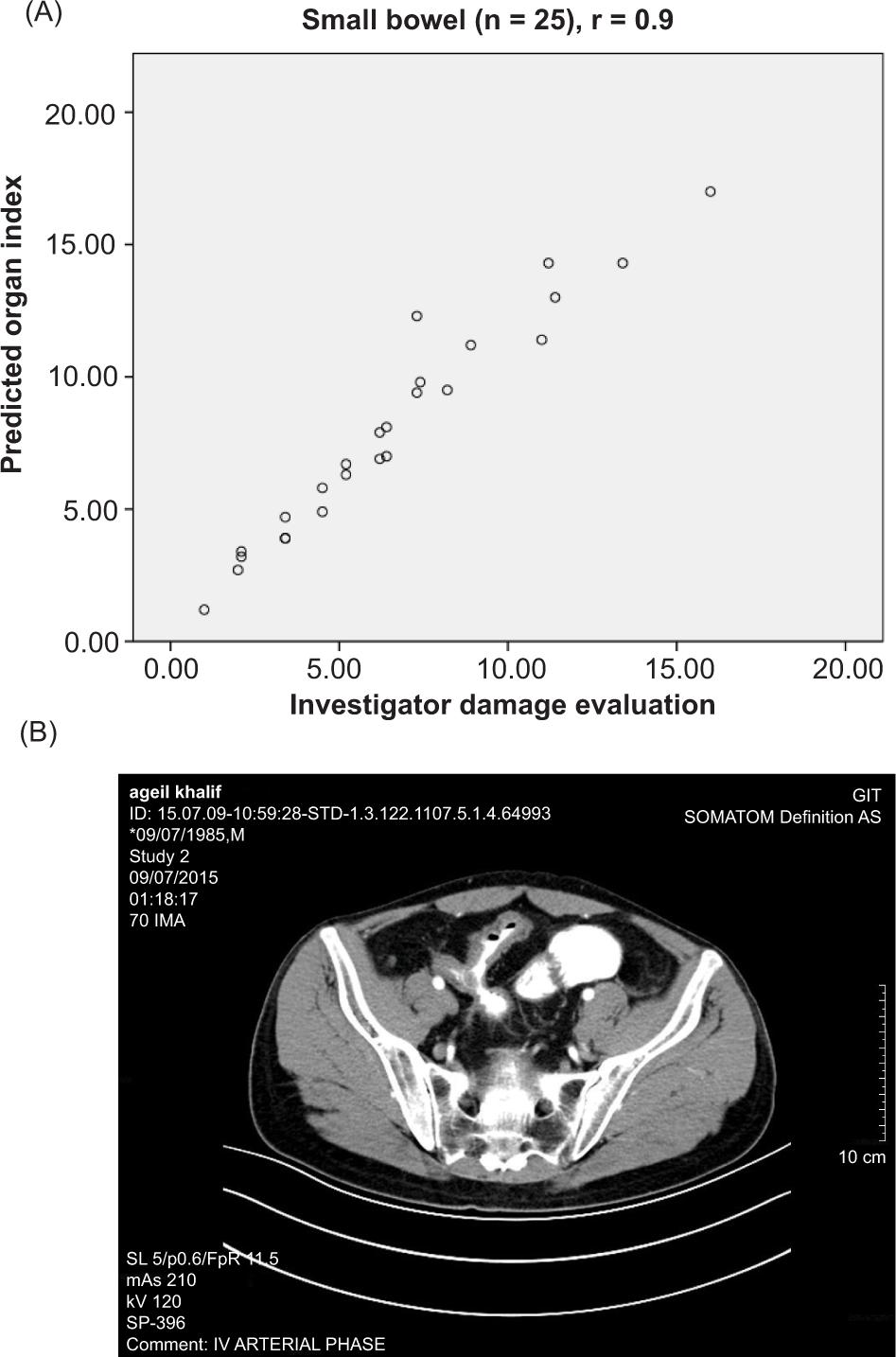
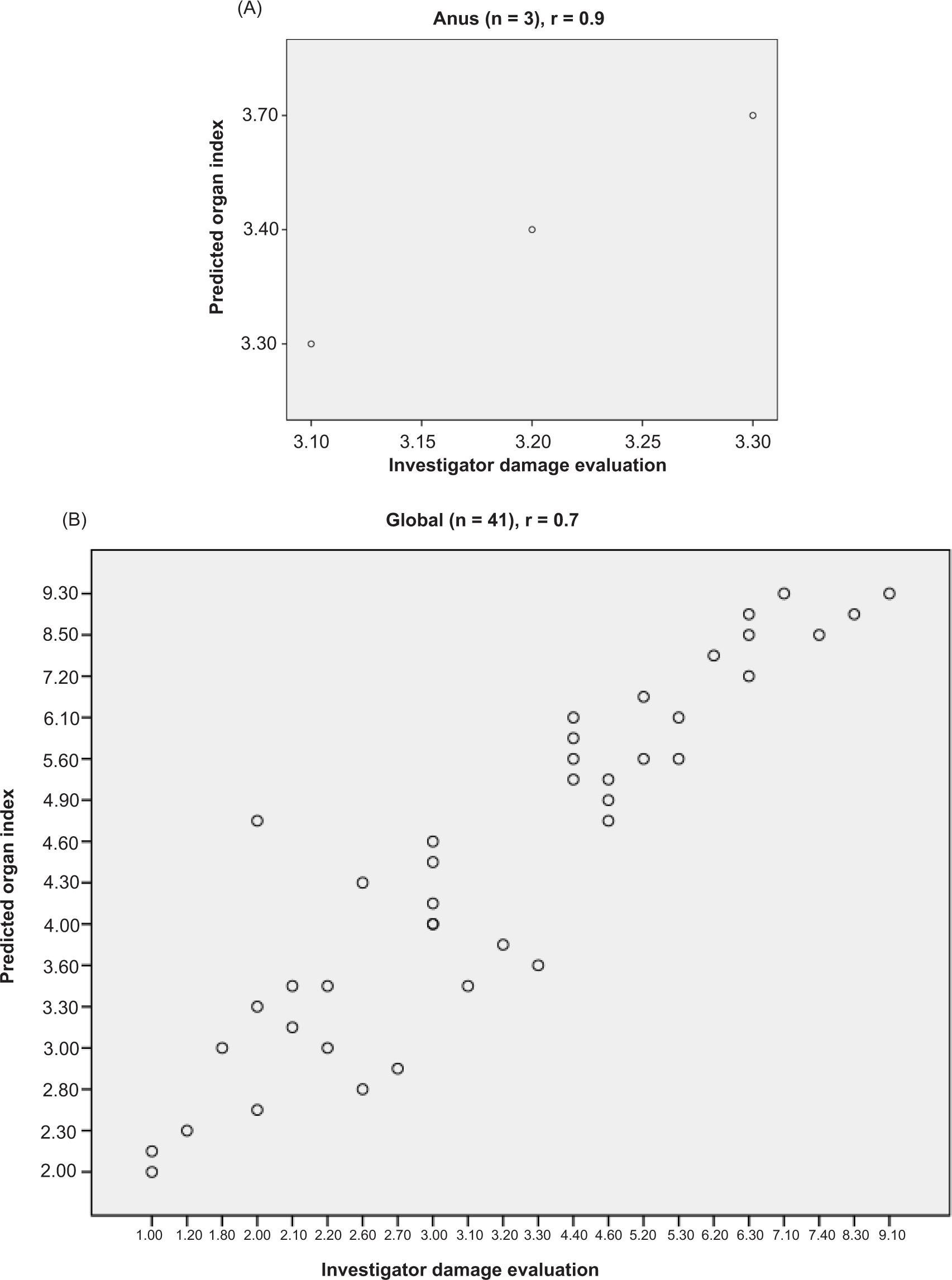
Figure 3. Scatterplot of the predicted indexes as a function of the investigator damage evaluations. (A) Organ indexes versus organ damage evaluation calculated from investigator segmental damage evaluations. (B) Global index versus investigator global damage evaluation.
Statistical analysis
For data entry and analysis, SPSS version 20 software was used. For the index construction, each predicted organ index was constructed through a multiple linear mixed model in the organ subsample composed of all patients with organ damage known or suspected at enrollment and all patients with organ damage found during. The dependent variable was the calculated organ damage evaluation. The independent variables of the model were the number of segments with stricturing lesions, regardless of their length, of each severity grade and the number of segments with penetrating lesions of each severity grade. The final coefficients of the organ damage index were derived from the estimated coefficients rounded to the nearest 0.5. The same method was used to construct the predicted global index using investigator global damage evaluation as the dependent variable, the four calculated organ damage evaluations as independent variables. The final coefficients of the LI were derived from the global index estimated coefficients multiplied by 10 and rounded to the nearest unity.
RESULTS
Organ damage evaluations were calculated as the sum of the segmental damage evaluations (resections excluded) for patients with known or suspected organ damage at enrollment: 0 for the upper digestive tract, 25 for small bowel, 13 for colon/rectum, and 3 patients for the anus. Mean (SD, range) organ damage evaluations were 0 (0, 0–0) for upper digestive tract, 6.2 (8.2, 0–74) for small bowel, 6.1 (5.9, 0–47) for colon/rectum, and 3.2 (1.9, 0–7.1) for anus. The global damage evaluation ranged from 0 to 9, with a mean (SD) of 1.74 (1.3).
For the 30 patients included, median LI was 1.3 (range 0.2–12.6). An example of the calculation of the index is provided in Table 4.
TABLE 1. Examples of the Scoring System Used when Calculating the Lémann Score: Severity Scale for Small Bowel Lesions According to the Lesions or History of Surgery or Any Other Interventional Procedures.
| Grade | Stricturing lesions | Penetrating lesions | History of surgery or any other interventional procedure |
|---|---|---|---|
| 0 | Normal | Normal | No procedure |
| 1 | Wall thickening <3 mm and/or segmental enhancement without prestenotic dilatation | Endoscopic dilatation | |
| 2 | Wall thickening ≥3 mm and/or mural stratification without prestenotic dilatation | Transmural fissure with increased density in perienteric fat | By-pass diversion or stricturoplasty |
| 3 | Stricture with prestenotic dilatation | Abscess or fistula | Resection |
TABLE 2. Distributions of Damage Components Including Number of Resected Segments or Number of Segments with Structuring or Penetrating Lesions of Most Severe Grade (N = 30).
| Organ (n= number of patients with organ involvement) | No. of segment | Resection | Without stricturing or penetrating lesion | Stricturing lesion with maximal grade | Penetrating lesion with maximal grade | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No | Yes | 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |||
| Upper tract | 1 | 30 | 0 | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Small intestine (n=25) | 1 | 30 | 0 | 5 | 5 | 0 | 0 | 0 | 20 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| >3* | 0 | 0 | 0 | 5 | 21 | 0 | 0 | 0 | 0 | 5 | ||
| Colon/rectum (n=13) | 1 | 30 | 0 | 17 | 17 | 0 | 7 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 0 | ||
| 3 | 0 | 0 | 0 | 2 | 3 | 0 | 0 | 0 | 0 | 0 | ||
| >3* | 0 | 0 | 0 | 0 | 11 | 0 | 0 | 0 | 0 | 1 | ||
| Anus (n=3) | 1 | 30 | 0 | 27 | 30 | 0 | 0 | 0 | 27 | 0 | 0 | 3 |
*Refers to the distal segments: 4–20 for small bowel and 4–6 for colon/rectum.
TABLE 3. Estimated and Final Coefficients to be Applied to the Number of Segments with Stricturing and Penetrating Lesions of Each Grade of Severity in Order to Calculate the Organ Indexes and the Calculated Organ Damage Evaluations in Order to Calculate the LI .
| Organ index | Type of lesion | Grade of severity | Coefficient | P-value | Final coefficient | |
|---|---|---|---|---|---|---|
| Estimate | S E | |||||
| Small intestine | Stricturing | G1 | 0.7 | 0.18 | 0.01 | 1 |
| G2 | 2.1 | 0.21 | 0.02 | 2 | ||
| Penetrating | G3 | 4.2 | 0.14 | 0.01 | 4 | |
| Colon/rectum | Stricturing | G1 | 1.02 | 0.24 | 0.01 | 1 |
| G2 | 1.6 | 0.34 | 0.01 | 1.5 | ||
| Penetrating | G3 | 4.1 | 0.41 | 0.01 | 4 | |
| Anus | Penetrating | G3 | 3.4 | 0.32 | 0.01 | 3.5 |
| Lemann index Calculated investigator organ damage evaluation | ||||||
| Small bowl | 0.43 | 0.06 | 0.03 | 4.5 | ||
| Colon/rectum | 0.32 | 0.03 | 0.01 | 3 | ||
| Anus | 0.30 | 0.01 | 0.02 | 3 | ||
TABLE 4. Example of LI Calculation: 31-year-old Male Presented with Stricturing Lesion (Grade 2) of the Terminal Ileum and Fistula Formation (Penetrating Lesion Grade 3) Between Terminal Ileum and Sigmoid Colon.
| Segment and organ | Resection | Stricturing lesions | Penetrating lesions | Index | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | Index | G1 | G2 | G3 | Index | ||||||||
| Esophagus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Stomach | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Duodenum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Upper tract (sum of segmental indexes/3) | 0 | ||||||||||||||
| Small bowel 1 | 0 | 0 | * | 0 | 2((Coefficient of stricturing lesion of small bowel of G2)) (2×1 segment) | 0 | 0 | * | 4(coefficient of penetrating lesion of small bowel G3 (4× 1 segment)) | 6 | |||||
| Small bowel 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Small bowel 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Small bowel 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Small bowel | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Small bowel 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Small bowel (sum of segmental indexes / 20) | 6/20(number of segments of small bowel) =0.3 | ||||||||||||||
| Cecum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Ascending colon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Transverse colon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Descending colon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Sigmoid colon | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Rectum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Colon/rectum (sum of segmental indexes/6) | 0 | ||||||||||||||
| Anus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||
| Anus (1 segment) | 0 | ||||||||||||||
| Lemann index | 0.3 × 4.5 (organ index of small bowel) =1.4 | ||||||||||||||
DISCUSSION
The LI is the first tool that measures cumulative bowel damage by using resections and the extent and severity of lesions in the digestive tract of CD patients. In contrast to other indexes that are based only on clinical or endoscopic data and assess the severity of inflammatory activity at a specific time and fluctuate with time, the LI is more likely to increase with the longer disease duration by assessing irreversible bowel damage along with accumulation of stricturing lesions, penetrating lesions, and surgical resections. Gilletta et al.18 evaluate the changes in LI values during the first years of CD. A value of 2 was chosen as the cut-off for substantial transparietal damage. Index scores were shown to increase at each stage of follow-up and nearly two-thirds have substantial bowel damage 2–10 years after diagnosis. The only early factor that predicted later damage was the first index value. Changes in LI with time may be used to stratify a patient’s disease into aggressive, indolent, or treatment responsive types. Further analysis in these groups will yield inputs on risk factors for rapid damage progression and prognostication, as well as on therapeutic strategies to try preventing the damage.19 An important point will be to identify in the short-term patients who progress rapidly to significant damage. If we can identify those patients during the first few months following diagnosis we can treat them aggressively with the more potent drugs (biologics) having the objective to delay or avoid surgery. Conversely, patients who do not develop (significant) damage or who do not progress may be treated with more classical drugs.20 In a retrospective study conducted by Bodini et al., 21 88 patients with CD were divided based on the drug administered during a median follow-up period of 26 months into three groups: Group A – biological mono-therapy; Group B – azathioprine therapy; Group C – mesalamine therapy. The LI at the beginning and end of the follow-up period. The study suggests that the use of biological therapy rather than azathioprine and mesalamine changes the cumulative structural damage to the bowel and, therefore, modifies the disease progression of CD, preventing its long-term associated disability. In another prospective observational cohort pilot study conducted by Fiorino et al.,22 CD-related bowel damage was graded at baseline before starting anti-TNF and then each year during a follow-up period of 3 years. The study suggests that LI might be able to detect the change in bowel damage and assess response to therapy and that anti-TNF may probably stop bowel damage progression in the long term, as assessed by the LI. While validation is pending, recent reports suggest that bowel damage is reversible by anti-TNF therapy and the LI may play a key role in CD management, and should be implemented in all upcoming disease-modification trials in CD.23
CONCLUSIONS
The LI is now available and it enables, for the first time, the assessment of bowel damage in CD.
RECOMMENDATIONS
-
Measurement of LI at initial presentation of CD and during follow-up for better identification of patients with severe damage and those with rapid progression of damage (Further studies are required to determine the optimal time for follow-up measurement).
-
The early implementation of a more aggressive treatment strategy with immunosuppressive and/or biologic agents for patients with high initial index value or those with a significant increase at each stage of follow-up.
-
The implementation of the index in all upcoming trials evaluating the effects of medical therapies or strategies on CD progression.