INTRODUCTION
Death of the fetus is one of the common unfortunate pregnancy outcomes in daily obstetrics practice worldwide.1 A variety of definitions and terms have been addressed to the death of a fetus during pregnancy based on scientific and legal issues; besides, the reporting requirements vary among countries and states. The parameters used for labeling definitions are also variable including clinical estimates of gestational age, estimates of body length and birth weight.2 Generally speaking, there are two common terms to describe fetal death, stillbirth and spontaneous abortion; however, these two terms differ in the timing at which the death occurs during pregnancy.
The recording of fetal death during clinical researches depends on the exact definition of the phenomenon of fetal death in relation to gestation. Indeed, there is no consensus about the exact term when describing fetal death worldwide, taking into consideration the two common terms – spontaneous abortion versus stillbirth. This is due to the variation in the reporting policy in various regions of the world along with the variation in the method of estimating gestational age.2,3 Therefore, it is difficult to exactly compare stillbirth rates among different countries because of variation in definitions and methodologies. The WHO criteria for third-trimester stillbirth included a weight of 1 kg or more at time of birth, completing 28 weeks of gestation or at least having 35 cm crown heal length.1
In the current study, stillbirth definition was based on gestational age so that any fetal death before completing 28 weeks was excluded in addition to exclusion of birth-associated fetal death.
The incidence of stillbirth is variable among different countries but, in general, ranges from 3.1 to 6.2 per 1000 births.1,4 Most of the reported cases of stillbirths come from underdeveloped countries.5–7 The adoption of antenatal strategies to care for high-risk women has significantly reduced the incidence of stillbirth in developed countries1; therefore, the identification of risk factors associating stillbirth, together with encouraging health facilities to deal with and manage such factors can greatly reduce the incidence of stillbirth in developing countries including ours. The under-reporting and poor registration of stillbirth in developing countries because of home deliveries may contribute further to unclear ideas about the true incidence rate of stillbirth in those countries.8–12
Causes of fetal death are often difficult to identify and it is important to make a distinction among mode of death, exact etiology of death, and its classification.13–15 In general, causes have been grouped into maternal,16 fetal,17–19 placental,20 and external factors.21
In fact, clear data about the exact annual incidence of stillbirth and its associated risk factors are lacking in our province, Al-Diwaniyah province, and the nearby regions of the mid-Euphrates region of Iraq. Therefore, the aim of the current study was to estimate the annual incidence rate of stillbirth in Al-Diwaniyah province and make an account of the principal risk factors associating stillbirth in this region of Iraq.
PATIENTS AND METHODS
The current cross-sectional study was carried out in Al-Diwaniyah Maternity and Children Teaching Hospital at Al-Diwaniyah province, the mid-Euphrates region of Iraq. This hospital receives most of the cases of delivery in the province because it is the only hospital dedicated to obstetrics in the province. The study included reviewing available birth records for a complete 1-year period starting from May 1, 2018 to April 30, 2019. Inclusion criteria included fetal death in women who completed 28 weeks of gestation. Fetal death that happened at or after delivery was excluded from the study.
Variables included in the current study were: maternal age, residency, level of education, socioeconomic status, gestational age, parity, antenatal care, previous intrauterine death, mode of delivery, blood group, fetal gender, pregnancy-induced hypertension, placental abruption, fetal congenital abnormalities, previous abortion, previous intrauterine death, and maternal anemia.
The study was approved by the institutional ethical approval committee and a formal agreement was issued by the directorate of health in the province, the formal representative of the Iraqi Ministry of Health. Data were transformed into an SPSS (statistical package for social science, version 16, IBM, Chicago, USA) spreadsheet for the purpose of statistical summarization, description, and analysis. The stillbirth incidence rate was calculated out of 1000 births. Qualitative variables were expressed in the form of numbers and percentages.
RESULTS
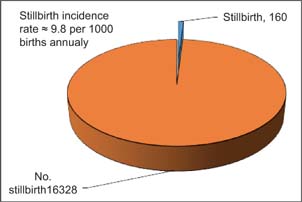
The total number of deliveries in Al-Diwaniyah Maternity and Children Teaching Hospital during the period of study was 16,328. The total number of antenatal stillbirth cases during the same period was 160; therefore, the annual incidence rate of stillbirth was approximately 9.8 per 1000 births, as shown in Figure 1.
FIGURE 1. Pie chart showing the annual incidence antenatal stillbirth rate in Al-Diwaniyah province, mid-Euphrates region of Iraq.
The sociodemographic characteristics of women enrolled in this study are shown in Table 1. Regarding maternal age, the majority of women were between 20 and 35 accounting for 129 (80.6%); women younger than 20 accounted for 10 (6.2%), and women older than 35 accounted for 21 (13.1%). Most of the women were from rural areas accounting for 93 (58.1%). The majority of women were either illiterate or completing only primary school, 65 (40.6%) and 73 (45.6%), respectively. Poor socioeconomic status was dominant followed by intermediate status and women with good status accounted for the minority of the enrolled sample, 99 (61.9%), 57 (35.6%), and 4 (2.5%), respectively.
TABLE 1. Sociodemographic characteristics of women enrolled in this study.
| Characteristic | Results |
|---|---|
| Maternal age (years) | |
| < 20, n (%) | 10 (6.2%) |
| 20–35, n (%) | 129 (80.6%) |
| >35, n (%) | 21 (13.1%) |
| Mean ±SD | 28.63 ± 6.66 |
| Range | 15–45 |
| Residency | |
| Urban, n (%) | 67 (41.9%) |
| Rural, n (%) | 93 (58.1%) |
| Level of education | |
| Illiterate, n (%) | 65 (40.6%) |
| Primary, n (%) | 73 (45.6%) |
| Secondary, n (%) | 18 (11.2%) |
| University, n (%) | 4 (2.5%) |
| Socioeconomic status | |
| Poor, n (%) | 99 (61.9%) |
| Intermediate, n (%) | 57 (35.6%) |
| Good, n (%) | 4 (2.5%) |
n: number of cases; SD: standard deviation.
Other maternal, fetal, and placental characteristics are shown in Table 2. Anemia was seen in a significant proportion of participating women (48.1%). The frequency distribution of women according to parity was as follows: 48 (30.0%) primiparous, 104 (65.0%) multiparous, and 8 (5.0%) grand multiparous women. Regular antenatal care was reported in a minority of women and lack of antenatal care or irregular one was reported in most of the cases, 3 (1.9%), 69 (43.1%), and 88 (55.0%), respectively. Cesarean section was seen in significant previous and present deliveries, 58 (36.2%) and 109 (68.1%), respectively. The proportion of women with Rh-negative blood group was 18 (11.2%). Pregnancy-induced hypertension was seen in 52 (32.5%) and diabetes mellitus was seen in 6 (3.8%). Previous abortion was seen in 18 (11.3%) and previous intrauterine death was seen in 5 (3.1%). Gestational age was ranging from 28 to 41 weeks with a mean of 33.42 ±2.99 weeks and there was a somewhat even distribution of cases according to gestational age; however, a rise in frequency was seen in weeks 32 through 37 (Table 3). Regarding fetal sex, there was an equal number of male and female sexes, 80 (50.0%) versus 80 (50.0%), respectively. Congenital abnormalities were seen in 19 (11.9%) and placental abruption was seen in 31 (19.4%).
TABLE 2. Maternal, fetal, and placental characteristics.
| Characteristic | Results |
|---|---|
| Anemia, n (%) | 77 (48.1%) |
| Parity | |
| Primiparous, n (%) | 48 (30.0%) |
| 1–5, n (%) | 104 (65.0%) |
| > 5, n (%) | 8 (5.0%) |
| Antenatal care | |
| No antenatal care, n (%) | 69 (43.1%) |
| Irregular, n (%) | 88 (55.0%) |
| Regular, n (%) | 3 (1.9%) |
| Previous delivery | |
| No previous delivery, n (%) | 50 (31.2%) |
| Normal vaginal delivery, n (%) | 52 (32.5%) |
| Cesarean section, n (%) | 58 (36.2%) |
| Mode of delivery | |
| Normal vaginal delivery, n (%) | 51 (31.9%) |
| Cesarean section, n (%) | 109 (68.1%) |
| Blood group | |
| Rh Positive, n (%) | 142 (88.8%) |
| Rh Negative, n (%) | 18 (11.2%) |
| Pregnancy induced hypertension, n (%) | 52 (32.5%) |
| Diabetes mellitus, n (%) | 6 (3.8%) |
| Previous abortion, n (%) | |
| Total, n (%) | 18 (11.3%) |
| Single, n (%) | 8 (5.0%) |
| Recurrent, n (%) | 10 (6.3%) |
| Previous intrauterine death, n (%) | 5 (3.1%) |
| Gestational age (weeks) | |
| Mean ±SD | 33.42 ± 2.99 |
| Range | 28–41 |
| Fetal sex | |
| Male, n (%) | 80 (50.0%) |
| Female, n (%) | 80 (50.0%) |
| Fetal abnormalities, n (%) | 19 (11.9%) |
| Placental abruption, n (%) | 31 (19.4%) |
n: number of cases; SD: standard deviation.
TABLE 3. Frequency distribution of women according to gestational age.
| Gestational age (weeks) | N | % |
|---|---|---|
| 28 | 11 | 6.9 |
| 29 | 4 | 2.5 |
| 30 | 15 | 9.4 |
| 31 | 11 | 6.9 |
| 32 | 22 | 13.8 |
| 33 | 16 | 10.0 |
| 34 | 25 | 15.6 |
| 35 | 16 | 10.0 |
| 36 | 18 | 11.2 |
| 37 | 10 | 6.2 |
| 38 | 5 | 3.1 |
| 40 | 5 | 3.1 |
| 41 | 2 | 1.2 |
DISCUSSION
In the current study, we tried to make an idea about the annual incidence rate of antenatal stillbirth in Al-Diwaniyah province, Iraq. In addition, in the current cross-sectional study, we tried to highlight questions about the principal risk factors responsible for antenatal stillbirth in our province. One of the most important limitations was that the study was neither a case control study nor a cohort study to know the magnitude of risk subjected by each enrolled factor in terms of odds ratio and relative risk. However, we were able to identify several risk factors that were seen in a significant proportion of women with antenatal stillbirth. In the current study, the annual incidence rate of stillbirth was approximately 9.8 per 1000 births. The incidence of stillbirth is variable among different countries but in general ranges from 3.1 to 6.2 per 1000 births.1,4 Therefore, the incidence rate in our study is relatively higher than other regions of the world. Lack of regular antenatal care was common in almost all participants. The observation of the link between lack of regular antenatal care and the risk of stillbirth has been raised by previous authors.22,23 When pregnant women do not keep regular antenatal care, there will be a significant number of maternal and fetal abnormalities to be missed because of a lack of regular history taking and examination. For example, maternal illnesses such as hypertension and diabetes can be missed and these may lead to complications causing intrauterine fetal death. Detection of fetal abnormalities early in the course of pregnancy may permit early intervention and reduces the risk of future stillbirth. Therefore, the lack of regular antenatal care appears to be an important risk factor for stillbirth and can be avoided by the adoption of strategies that encourage pregnant women to keep regular antenatal care.
On the other hand, poor socioeconomic status and low level of education were very frequent in our study. Previous studies have demonstrated a significant association between sociodemographic factors and the risk of fetal death.24,25 A low level of education was found to be one of the independent risk factors of intrauterine death.23 Poverty is a feature of underdeveloped countries and most of the reported cases of stillbirths come from underdeveloped countries.5–7 Therefore, economic strategies should be adopted by the government in order to reduce the proportion of poor families in our community, as poverty is associated with other important health issues in addition to obstetric adverse outcomes. In the current study, most of the participants were between 20 and 35 years of age and a minority of them were younger than 20 years or older than 35 years. This finding may contradict the previous belief that the extremity of maternal age may raise the risk of stillbirth.26–28 In the current study, weeks 32 through weeks 37 had the highest frequency of women with stillbirth. Weeks 37 through 41 were the main implicated weeks in the study of Reddy et al.,26 and weeks 37 through 39 were the main weeks implicated in stillbirth in the study of Lavin and Pattinson23; thus, it appears that even when the fetus reaches term, the risk of death is greater, indicating that some intrinsic fetal abnormality of some increasing maternal pathology is responsible for intrauterine fetal death and not merely the low gestational age. In the current study, cesarean section was the dominant mode of present delivery and contributes significantly to the mode of delivery in the previous baby. It has been found that delivery by cesarean section in the first pregnancy could increase the risk of unexplained stillbirth in the second. “In women with one previous cesarean delivery, the risk of unexplained antepartum stillbirth at or after 39 weeks’ gestation is about double the risk of stillbirth or neonatal death from intrapartum uterine rupture.”29 However, other authors have found no significant association between previous cesarean section and present stillbirth.21 So far, it appears that the association between cesarean section and risk of stillbirth is still controversial and much research work is needed in order to reach a clear consensus about such association. Pregnancy-induced hypertension was a common observation in the current study. Several studies have demonstrated the significant association between maternal hypertension whether pregnancy-induced or chronic with the risk of stillbirth.30,31 Therefore, regular antenatal care will certainly permit the early recognition and proper treatment of hypertension in pregnant ladies thereby reducing the pregnancy-associated maternal and fetal complications accompanying hypertension. A significant proportion of women in the current study has placental abruption and fetal abnormalities. Both fetal abnormalities and placental abruption are shown to be significantly associated with stillbirth by previous authors.32–34 These two risk factors can be detected and managed properly if regular antenatal care is kept by pregnant women.
CONCLUSIONS
In conclusion, the annual incidence rate of stillbirth is relatively high in comparison with nearby countries and other regions of the world. Lack of regular antenatal care, poverty, maternal anemia, and pregnancy-induced hypertension appear to be the principal risk factors of antenatal stillbirth in our province.