INTRODUCTION
Type-2-diabetes mellitus is now considered the new plague of the current century, and both its incidence and prevalence are rapidly increasing worldwide.1,2 This global epidemic is primarily driven by obesity and unhealthy dietary habits, including excessive calorie intake and an unbalanced nutrient composition.3–5
Chronic insulin resistance and a progressive decline in beta-cell function are discussed as the root causes of type-2-diabetes.6–8 Both were linked to obesity and frequently associated with elevated concentrations of circulating free fatty acids (FFA) in the blood.9 The harmful effects of chronically elevated FFA levels on glucose homeostasis and non-adipose tissues are referred to as lipotoxicity.9–11
It is now widely accepted that prolonged exposure to elevated FFA levels in the presence of hyperglycemia contributes to beta-cell damage.9,11 In fact, fatty acid derivatives were shown to interfere with beta-cell function and ultimately lead to their death through lipoapoptosis.11,12
A recent review suggested that both FFA quality and quantity impact beta-cell function.9 Several studies found that diets high in saturated lipids are particularly harmful to beta-cells.13–15 According to Hagman, prolonged exposure to palmitate, an ester of the saturated palmitic acid, impairs insulin gene expression and increases beta-cell apoptosis.6,16 Moreover, excessive accumulation of cholesterol appears to play an important role in beta-cell function.17,18 The increased uptake of low-density lipoprotein cholesterol by islet beta-cells and subsequent oxidative reactions were shown to be detrimental for these cells.17,19
Unsaturated fatty acids, on the other hand, were associated with a variety of beta-cell protective effects, including prevention of beta-cell apoptosis and enhanced insulin sensitivity.14,20,21 Furthermore, supplementation of n-3 PUFA for human subjects was shown to enhance insulin secretion and to prevent both beta-cell destruction and insulin resistance.20 In a Japanese cohort, a higher intake of plant-derived alpha-linolenic acid (ALA) was significantly associated with a lower prevalence of insulin resistance in normal-weight individuals.22
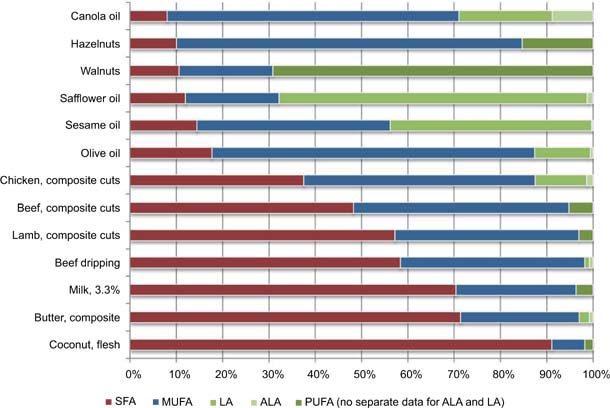
While fatty acids are essential components of the human diet, they can be obtained from two main sources: plants and animals.14 Animal fats usually contain high amounts of saturated fatty acids (SFA)14,23 while lipids from plants are often rich in monounsaturated fatty acids (MUFA) and PUFA.24,25 The fatty acid profiles of common fats and oils are shown in Figure 1.26,27
FIG 1. Fatty acid profiles of dietary fat present in selected foods. SFA: saturated fatty acids; MUFA: monounsaturated fatty acids; LA: linoleic acid; ALA: α-linolenic acid; PUFA: polyunsaturated fatty acids. Note: where separate data for ALA and LA was available it was used, otherwise total PUFA was used. With friendly permission of the New Zealand Nutrition Foundation.
Vegetarians and vegans, who avoid or completely restrict animal products, were shown to have a lower overall risk of common chronic diseases, including obesity, hypertension, type-2-diabetes, and heart disease.28–31 This is possibly due to lower cholesterol and saturated fat intake and much higher consumption of dietary fiber in contrast to omnivorous diets.32,33
This raises the question of whether a dietary modification toward a low-fat vegan diet, which is particularly low in saturated and trans-fats, could reduce lipotoxicity, and thus improve beta-cell function in patients suffering from type-2-diabetes?
To investigate this hypothesis, this narrative review examined how a low-fat vegan diet specifically affected clinical parameters that may reduce lipotoxicity and contribute to improved beta-cell function. The available low-fat vegan intervention studies in individuals with type-2-diabetes were therefore analyzed for the following endpoints: (a) changes in total energy intake and total fat intake (fat quantity), (b) changes in fat quality (saturated vs unsaturated fat intake), (c) changes in adipose tissue mass and weight reduction, and (d) changes in glycemic control with a low-fat vegan diet. Only studies that reported on at least one of the above-mentioned endpoints were considered eligible for this review.
METHODS
To identify relevant studies for this narrative review, the electronic database of PubMed was searched using the keywords “Diabetes”, “Plant-based”, “Vegetarian”, and “Vegan”, combined into the following query: “(vegan OR vegetarian OR plant-based) AND diabetes”. The filter “humans” was applied.
Only English language articles were included in this narrative review. Original articles, reviews, and commentaries were screened. Additionally, cross-references and reference lists of the included articles were manually checked for additional content to ensure that all potentially relevant studies were identified. No time restriction was applied. The entire review process was conducted by the author (MAS). The Academy of Nutrition and Dietetics’ narrative review checklist is available for download as a supplementary file.
Studies were included when they reported upon a low-fat vegan dietary intervention in individuals suffering from type-2-diabetes. Hereby, a low-fat vegan diet was defined by a (planned) macronutrient distribution of approximately 75% of energy from carbohydrates, 15% from protein, and 10% from fat. Only studies with a minimum duration of 4 weeks were included. Furthermore, studies were also included in this review when the dietary intervention was combined with other lifestyle modifications, for example, meditation, exercising, or behavior therapy.
Studies containing foods that are technically vegan but highly processed (e.g., formula drinks) were excluded from this review. Macrobiotic diets that occasionally allow for small amounts of milk and fish were excluded from this review as well.
RESULTS
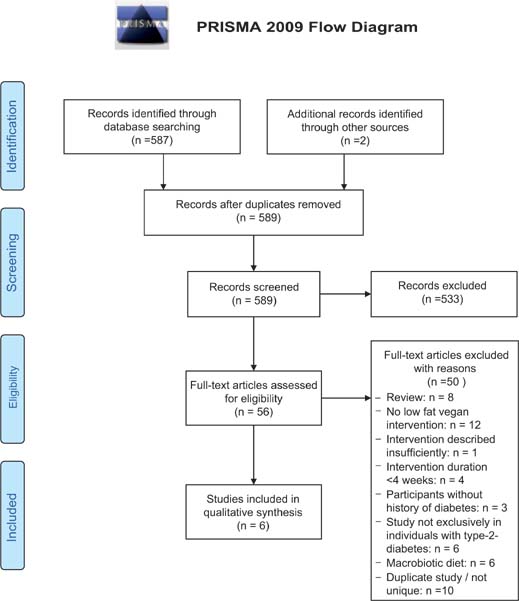
The initial PubMed search yielded 587 articles published between 1967 and September 2020. In addition to that, two articles were found after screening the reference lists of the included articles. A total of 589 references were screened. Five hundred and thirty-three articles were excluded after reading titles and abstracts based on the pre-defined eligibility criteria. Fifty-six articles remained eligible for full-text review. After full-text review, 50 articles were excluded (Figure 2).34
FIG 2. PRISMA 2009 flow diagram.
Twelve studies reported upon a low-fat vegan dietary intervention in individuals suffering from type-2-diabetes.35–46 Six of these studies, however, did not exclusively recruit patients with type-2-diabetes but also included individuals with other diseases, including hypertension, coronary artery disease, and obesity.41–46 These studies were not included in the “Results” section of this narrative review because persons with diabetes represented fairly small subgroups in these trials.
After the exclusion of these studies, six trials remained eligible for this review.35–40 All of these studies reported on at least one of the endpoints under discussion, including (a) changes in total energy intake and total fat intake, (b) changes in fat quality, (c) changes in adipose tissue mass and weight reduction, and ultimately (d) glycemic control with a low-fat vegan diet. The findings of this narrative review are presented in this exact sequence below.
Table 1 lists the eligible studies using a low-fat vegan dietary intervention in patients with type-2-diabetes. The results of this narrative review are presented under four subsections, including (a) changes in total energy intake and total fat intake, (b) changes in fat quality, (c) changes in adipose tissue mass and weight reduction, and (d) improved glycemic control with a low-fat vegan diet.
TABLE 1. Changes in Total Energy and (Saturated) Fat Intake across Intervention Studies Using a Low-fat Vegan Diet in Individuals with Type-2-Diabetes.
| Authors (year) | Location | Type | Duration (weeks) | Description of the intervention | Control group / Comparator | Participants in the intervention group (m, f, t) | Mean age (years) | Changes in total fat intake (% of energy) | Changes in saturated fat intake (% of energy) | Changes in total energy intake (kcal) |
|---|---|---|---|---|---|---|---|---|---|---|
| Nicholson et al. (1999)35 | United States of America | RCT | 12 |
|
|
4, 3, 7 | 51 | –23 Baseline: 34 ± 5.3 Final: 11 ± 4.7 |
–7 Baseline: 10 ± 2.4 Final: 3 ± 2.0 |
–274 Baseline: 1683 ± 435 Final: 1409 ± 549 |
| Barnard et al. (2009)36 | United States of America | RCT | 74 |
|
|
22, 27, 49 | 56.7 (±9.8) | –14 ± 1.8 Baseline: 36.3 ± 1.3 Final: 22.3 ± 1 |
–6.7 ± 0.7 Baseline: 11.9 ± 0.5 Final: 5.1 ± 0.5 |
–432 ± 81 Baseline: 1798 ± 72 Final: 1366 ± 81 |
| Bunner et al. (2015)37 | United States of America | RCT | 20 |
|
|
6, 11, 17 | 57 (±6) | n/a | n/a | n/a |
| Lee et al. (2016)38 | Republic of Korea | RCT | 12 |
|
|
6, 40, 46 | 57.5 (±7.7) | n/a Mean fat intake (g) during intervention period: 31.8 ± 6.3 |
n/a Mean saturated fat intake (g) during intervention period: 3.2 ± 1.5 |
n/a Mean energy intake (kcal) during intervention period: 1496.2 ± 104.8 |
| Ramal et al. (2017)39 | United States of America | RCT | 26 |
|
|
4, 13, 17 | 53.35 (±6.74) | n/a | n/a | n/a |
| Barnard et al. (2018)40 | United States of America | RCT | 20 |
|
|
8, 13, 21 |
61 | –15 ± 2 Baseline: 33 ± 2 Final: 18 ± 2 |
–7 ± 1 Baseline: 10 ± 1 Final: 4 ± 1 |
–204 ± 95 Baseline: 1695.1 ± 129 Final: 1491 ± 129 |
m = male; f = female; t = total.
All values refer to changes in the intervention groups using a low-fat vegan diet.
Changes in total energy intake and total fat intake
Total energy intake was restricted in none of the studies under discussion. Nevertheless, a dietary modification toward a low-fat vegan diet altered both total energy and fat intake across all studies (Table 1).
As shown in Table 1, total energy intake decreased consistently across studies. The most pronounced reduction was observed in a trial by Barnard and colleagues.36 The authors compared a low-fat vegan diet to a conventional diabetes diet, based on the 2003 American Diabetes Association guidelines. After 74 weeks on a low-fat vegan diet, participants significantly reduced energy intake. The mean total energy intake dropped by 432 ± 81 kcal (Table 1).
Several years later, the same study group published another study comparing a low-fat vegan diet to a portion-controlled diet.40 In this 20-week randomized clinical trial, the mean total energy intake dropped by 204 ± 95 kcal in the low-fat vegan group. Comparable results were observed in another study by Nicholson and colleagues,35 that compared a low-fat vegan diet to a conventional low-fat diet. While neither animal foods nor refined carbohydrates were allowed in the vegan group, the control diet emphasized the use of fish and poultry instead of red meat. After 12 weeks, the mean total energy intake decreased from 1683 ± 435 kcal at baseline to 1409 ± 549 kcal in the low-fat vegan group.
A 2016 Korean study compared a vegan diet to a conventional diet recommended by the Korean Diabetes Association.38 The vegan diet consisted of whole grains, vegetables, fruits, and legumes. Animal foods (i.e., meat, poultry, fish, dairy goods, and eggs) and processed foods of rice flour or wheat flour were not allowed in the vegan group. The authors did not report upon relative changes but found that mean energy intake during the intervention period was 1496.2 ± 104.8 kcal in the vegan group. Three studies did not report quantitative data on changes in total energy and fat intake.37–39
The percentage of energy intake from fat decreased substantially across studies as well (Table 1). Two studies by Barnard et al. reported a reduction of 14 and 15%, respectively.36,40 This reduction was even more pronounced in a pilot study by Nicholson et al.35 Lee and colleagues39 reported a mean fat intake of 31.8 ± 6.3 g in their vegan intervention group during the 12-week intervention period. Most importantly, the percentage of energy from saturated fat decreased significantly (Table 1) across studies, reflecting not only a change in fat quantity but also in fat quality.
Changes in fat quality
A dietary modification toward a low-fat vegan diet led to a significant reduction in saturated fat intake (Table 1). In all studies that investigated saturated fat intake,35,36,40 participants were able to reduce their saturated fat intake to <7% of total calorie intake. As such, participants were able to meet the current recommendations of the American Heart Association, which recommends a reduction of saturated fat intake to <7% of total calorie intake.
In addition to that, one study revealed a significant reduction in trans-fat intake.36 Participants in the vegan intervention group cut their trans-fat intake by more than 50% (from 2.3 ± 0.2% of energy at baseline to 1.1 ± 0.1% of energy at the end of the study).
Since low-fat vegan diets contain neither animal fat nor cholesterol, daily cholesterol intake decreased dramatically. In 2018, Barnard et al.40 reported a mean reduction of 169 ± 16 mg (from 174 ± 17 mg to 5 ± 2 mg). In an earlier study by the same group of researchers, a low-fat vegan diet led to a reduction in cholesterol intake of 122.7 ± 16.9 mg/1000 kcal. The most pronounced reduction in daily cholesterol intake was observed by Nicholson et al.35 (from 289 ± 86 mg at baseline to 4.4 ± 7.4 mg after 12 weeks). Lee and colleagues39 reported a mean cholesterol intake of 70.3 ± 57.4 g in their vegan intervention group.
Changes in body weight and adipose tissue mass
Low-fat vegan dietary interventions also have a substantial impact on body weight. Figure 3 illustrates changes in body weight among participants with type-2-diabetes who were assigned to a low-fat vegan diet for at least 1 month.
FIG 3. Weight loss with a low-fat, vegan diet in patients suffering from type-2 diabetes. A review of the current studies.
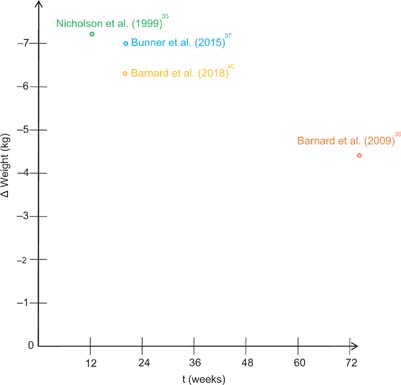
In a study by Nicholson et al.,35 a low-fat vegan diet led to an average weight loss of 7.2 kg within 12 weeks. In 2015, Bunner et al.37 reported comparable results. The authors investigated the effects of a low-fat vegan dietary intervention in patients with type-2-diabetes and chronic diabetic neuropathy pain. Participants who were assigned to a low-fat vegan diet lost 7 kg within 20 weeks. A study by Barnard and colleagues36 demonstrated that vegan-diet-induced weight loss may be sustained over a period of 74 weeks, indicating successful long-term results.
In a more recent study, Ramal et al.39 investigated the impact of a plant-based (vegan) diet in Latinos with type-2-diabetes living in a medically underserved area in California (United States). The authors paired a low-fat plant-based-diet with intensive lifestyle support focusing on group interventions. After 6 months, however, no significant changes were observed regarding body mass index. In contrast to these findings, Lee et al.38 emphasized that a brown-rice based vegan diet leads to a significant reduction in body mass index in patients with type-2-diabetes. Participants who were assigned to a vegan diet reduced their body mass index by 0.5 ± 0.9 kg/m2 within 12 weeks.
In both studies, the dietary intervention lead to a notable decrease in waist circumference. Ramal et al.39 reported a decrease from 107.54 ± 3.09 cm to 104.82 ± 2.78 cm. In the Korean study, waist circumference also decreased by 3.1 ±4.9 cm (from 85.0 ± 9.8 cm at baseline to 81.9 ± 9.9 cm after 12 weeks).
Changes in glycemic control
Glycemic control significantly improved with a low-fat vegan diet. In a study by Barnard and colleagues,36 fasting plasma glucose levels decreased significantly with a low-fat vegan diet (from 163.5 ± 7.6 mg/dl at baseline to 144 ± 7.7 mg/dl after 74 weeks). A comparable reduction was observed by Lee et al.38 using a brown-rice based vegan diet: fasting plasma glucose levels dropped from 138.4 ± 52.4 mg/dl at baseline to 117.3 ± 32.1 mg/dl after 4 weeks. Bunner et al.37 reported a mean fasting plasma glucose reduction of 25.9 mg/dl after 20 weeks (from 160.6 ± 73.5 mg/dl at baseline to 134.6 ± 51.6 at the end of the intervention).
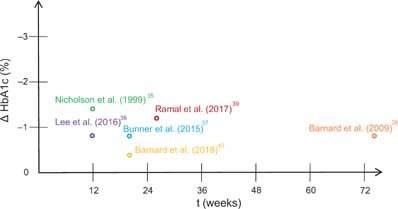
As shown in Figure 4, hemoglobin A1c, a long-term-marker for glycemic control,47,48 also improved significantly across studies. The most pronounced reductions were observed in studies by Nicholson et al.35 and Ramal et al.39 (Figure 4). In 2016, Lee et al.38 reported a mean HbA1c reduction of 0.9% (±0.8) in those patients with high compliance. A comparable reduction (–0.82%) was observed earlier by Barnard et al.36 among medication-stable participants in the vegan group after 74 weeks. In another study by the same group, a low-fat vegan intervention lead to a smaller HbA1c reduction (–0.4%). It must be noted, however, that median HbA1c values were below 7.0% in the intervention group at baseline in this study, which may have limited the potential for further reductions.
FIG 4. HbA1c reductions (in %) with a low-fat vegan diet in patients from type-2-diabetes. For Lee et al.,37 only patients with high compliance are displayed. For Barnard et al.,35 only medication-stable participants are displayed.
DISCUSSION
A dietary modification toward a low-fat vegan diet beneficially affected glycemic and weight control in patients with type-2-diabetes (Figures 3 and 4). Mean fasting plasma glucose levels and HbA1c-levels improved significantly across studies. Moreover, a vegan dietary intervention led to a significant reduction in total energy intake and total fat intake. Daily cholesterol intake and saturated fat intake decreased as well.
These changes might favorably affect beta-cell function and reduce lipotoxicity. Hereby, the significant reduction in mean daily saturated fat intake appears to be of paramount importance.
An oversupply of saturated fat was shown to be particularly harmful to beta-cells.13–15 It is conceivable that a reduction in saturated fat intake might reduce circulating levels of FFA. This could reduce the systemic pressure on cells and tissues to increase their lipid uptake and, in turn, reduce consecutive lipotoxicity. Hyperlipidemia imposes chronic insults on beta-cells via the generation of intracellular cytotoxic metabolites and activation of detrimental signaling pathways.12,49 Thus, tackling unfavorable lipid profiles in the first place appears to be of utmost importance.
Plant-based and vegan diets were repeatedly shown to exert beneficial effects on lipid control.50,51 As demonstrated in this narrative review, saturated fat intake decreases significantly with a low-fat vegan diet. Furthermore, a meta-analysis by Yokoyama and colleagues51 revealed that consumption of a vegan diet is associated with decreased low-density lipoprotein cholesterol and decreased total cholesterol. Since excessive accumulation of cholesterol appears to play an important role in impaired beta-cell function,17 a vegan diet could offer protective potential here.
The fatty acid composition is a critical factor in the induction of lipotoxicity in beta cells.52 Apart from reduced saturated fat intake, an optimized intake of PUFA could offer additional benefits. The latter were associated with several beta-cell protective effects, including enhanced insulin sensitivity and secretion as well as prevention of beta-cell apoptosis.14,20,21 A recent study suggested that PUFA intake, expressed as a percent of total fat intake, actually increases with a low-fat vegan diet.53 The relative consumption of linoleic (LA) and ALA, as a proportion of total fat, significantly increased with a low-fat vegan diet in overweight individuals in this study.
Another study examined the effects of a calorie-restricted, vegetarian (quasi-vegan) diet in combination with exercise on insulin resistance, oxidative stress markers, and visceral fat in patients with type-2-diabetes.54 When diet alone lead to significant improvements with regard to all three endpoints, the study also revealed a significant increase in PUFA to SFA ratio. A 2015 observational study from Denmark revealed comparable results: in comparison to the general Danish population, the ratio of PUFA to SFA appears to be more favorable in those following a vegan diet.55
Since substituting unsaturated (monounsaturated and/or polyunsaturated) fat for saturated fat in a persons’ diet appears to increase insulin sensitivity,56 a low-fat vegan diet and its favorable fatty acid composition might be of particular importance in type-2-diabetes.
Another factor that might contribute to improved beta-cell function is diet-induced weight loss.57,58 It is now widely accepted that obesity and excessive calorie intake are both associated with elevated concentrations of FFA.9,12,59,60 At first, the expanded adipose tissue mass is likely to release more FFA into the bloodstream. Secondly, clearance of FFA may be reduced due to an increased escape from esterification in adipose tissue.9,60,61 Finally, once plasma FFA levels are markedly elevated, they will inhibit the antilipolytic action of insulin, which is likely to increase the rate of FFA release into the circulation.60 Thus, it is conceivable that a reduction in adipose tissue mass might reduce circulating FFA levels.
As shown in this narrative review and by others, low-fat vegan diets induce weight loss by reducing total energy intake and energy density.62 They avoid animal products (which are high in saturated fat and cholesterol) and restrict industrially processed foods, which are often characterized by a high-calorie density.63,64 Therefore, low-fat vegan diets have been frequently associated with weight loss and a reduction in visceral fat.65–67 In this context, a more recent study by Kahleova et al.68 investigated the effects of a plant-based diet on body composition and insulin resistance. The authors demonstrated that a plant-based diet elicited changes in the gut microbiome that were associated with significant weight loss and a reduction in both total fat mass and visceral fat volume. It must be taken into account that none of the participants in this study suffered from type-2-diabetes.
However, considering the findings from this narrative review, individuals with type-2-diabetes also experienced significant weight loss with a low-fat vegan diet. As shown in Figure 3, participants allocated to the low-fat vegan diet lost up to 7.2 kg within 3 months. Most importantly, one study demonstrated that weight loss may be sustained over a period of 74 weeks, indicating successful long-term results.36
Another study published in 2015 also deserves mentioning here: a randomized controlled trial of five different diets suggested that vegan diets were more effective for weight loss than other diets.69 Vegan and plant-based diets appear to reduce body fat through a variety of mechanisms, which cumulatively lead to increased energy expenditure and a reduced calorie intake.70 It is conceivable that this reduction in body weight and visceral fat prevents or reduces lipotoxicity, simply because less FFA are released into the bloodstream due to a decrease in adipose tissue mass.
Diet-induced weight loss has already been shown to reduce the amount of other potentially lipotoxic metabolites, for example, excessive intramyocellular lipid accumulation.71 The latter refers to the ectopic deposition of FFA in muscle cells, which ultimately results in insulin resistance.72–74 A low-fat vegan diet has been frequently suggested to reduce intramyocellular lipid accumulation,70,75 indirectly indicating that lipotoxic effects can be modified with dietary modification.
Ultimately, this narrative review found that low-fat vegan diets led to significant improvements in glycemic control. HbA1c-values (Figure 4) and fasting plasma glucose levels significantly improved across studies. This is of paramount importance because the concurrent exposure to pathologically elevated glucose levels, also called glucotoxicity, is considered to exert synergistic toxic effects with abnormal levels of FFA.9 It is now becoming more and more clear that lipotoxic events are tightly coupled to excess glucose levels.76 Some authors even suggest that hyperglycemia may be a prerequisite for lipotoxicity.76 This concept termed glucolipotoxicity was proposed first by Prentki and Corkey77 and Poitout and Robertson.78
Low-fat vegan diets, which significantly improve glycemic control, could therefore be a key tool in reducing lipotoxicity-induced beta-cell dysfunction. As demonstrated here, many individuals with type-2-diabetes may achieve improved glycemic control or even normoglycemia after modifying their diet. Therefore, a low-fat vegan diet might help to eliminate one potential prerequisite for lipotoxic events to occur.
In summary, a dietary modification toward a low-fat vegan diet could reduce lipotoxicity, and thus improve beta-cell function in patients suffering from type-2-diabetes. This review revealed several potential mechanisms of action, including (1) reduced total fat intake, (2) improved fat quality, (3) improved body weight and a reduction in adipose tissue mass, and finally (4) improved glycemic control. These four mechanisms are likely to contribute complementarily to improved beta-cell function in patients suffering from type-2-diabetes. Physicians must consider these findings when counseling patients on lifestyle modifications and healthy nutrition.
It is important to note that this narrative review has several important limitations. The number of randomized studies in this particular field is limited. Beta-cell function was not routinely assessed in the studies under discussion and clinical trials, which specifically investigated the effects of a low-fat vegan diet in individuals with severely impaired and lipotoxicity-induced beta-cell function, are missing.
Moreover, this review has methodological limitations. Given this narrative review is a single author contribution, only one person screened and extracted the included literature. However, single-reviewer abstract screening was recently shown to miss approximately 13% of relevant studies.79 One may not exclude that some studies in the field were missed. Additionally, only one major electronic database (PubMed) was searched; therefore, it is possible that relevant studies from other sources might have been missed as well. With the literature search strategy, it must be noted that the exclusion criteria were quite strict. Studies that did not exclusively recruit patients with type-2-diabetes but also included individuals with other diseases, including hypertension, coronary artery disease, and obesity, were excluded from this review. Therefore, only six studies were included in this narrative review.
While a low-fat vegan diet beneficially affects glycemic and weight control in patients with type-2-diabetes, further research is necessary with its precise impact on beta-cell health and lipotoxicity. A 2018 study tested the effect of a vegan dietary intervention on beta-cell function and insulin resistance in overweight adults with no history of diabetes.80 A comparable study would be desirable in individuals with type-2-diabetes. Future studies including low-fat vegan diets that focus specifically on pancreatic beta-cell function would be of particular help. These studies could include both the more conventional beta-cell function measurements, such as the homeostasis model assessment and rather novel biomarkers including adiponectin and endocan.81,82 Such studies would help tremendously to confirm the beneficial effects of low-fat vegan diets on beta-cell function.
CONCLUSIONS
A progressive decline in beta-cell function in the presence of insulin resistance is now considered one of the root causes of type-2-diabetes. Prolonged exposure to elevated FFA levels in the presence of uncontrolled hyperglycemia contributes to impaired beta-cell function and ultimately beta-cell death through lipoapoptosis. This narrative review suggests that a dietary modification toward a low-fat vegan diet might help to counteract this process. By cutting the oversupply of saturated fat and by improving both body weight and glycemic control, low-fat vegan diets may reduce the likelihood of lipotoxic events to occur.