INTRODUCTION
The prevalence of epilepsy has been estimated to be 0.3–1% among pregnant women.1,2 Epilepsy and the control of seizures are often associated with major clinical challenges during pregnancy.3 Current epilepsy guidelines recommend managing epilepsy in pregnant women using the lowest effective dose of the most appropriate antiepileptic drugs (AEDs).4,5 Besides their use for managing epilepsy, AEDs are used in pregnant women for the treatment of pain syndromes, psychiatric disorders, and chronic migraine. Generally, the use of AED during pregnancy is associated with increased risks to the developing fetus, as these drugs can cross the placenta or be transferred to the infant through breast feeding, leading to an increase in adverse perinatal and neurodevelopmental outcomes.6 There is a consensus that AEDs in general introduce a risk of abnormal or delayed physical and neurodevelopmental effects among infants who are exposed during their vulnerable fetal developmental stages.3,4,7–11
The association between the use of AEDs during pregnancy and increased fetal risks has been well reported in literature; however, the comparative safety of AEDs was only highlighted in recent years.2,7,9,12,13 The use of AEDs during pregnancy is associated with increased rates of perinatal and cognitive adverse effects among the offspring, but those risks vary according to the type of AED, the combination of AEDs prescribed, and the drug dosages.9,14–16 Therefore, it is crucial to understand how AEDs affect long-term outcomes in the offspring.
AEDs have different mechanisms of action, and in turn, physical and neurodevelopmental risks of their in-utero exposure will vary.3,5 This is further compounded by the fact that the number of AEDs has increased substantially in the last 30 years. While evidence on the safety of lamotrigine has been growing, fewer data are available for other new-generation AEDs, such as levetiracetam and topiramate.7,9,17,18 Even less is known about the effect of new-generation AEDs on neurodevelopmental outcomes.7
Childhood neurodevelopmental disorders are a group of conditions that occur in the developmental period, often before children reach school age.19–21 Attention-deficit/hyperactivity disorder (ADHD) is the most common neurodevelopmental disorder in children, with an estimated worldwide prevalence around 5%.22,23 ADHD is a complex disorder that can affect individuals across the lifespan.22 As for other mental health conditions, there has, over the past two decades, been an increasing body of research on ADHD.24 Reasons for this upsurge include increased recognition of the impact of ADHD on functioning, advances in research methodology and technology, and a burgeoning interest from pharmaceutical companies.19,24,25 To date, there is no currently published comprehensive and updated review that summarizes the available evidence on the effect of new-generation AEDs on neurodevelopmental adverse outcomes, specifically ADHD. The aim of this scoping review was to summarize the published evidence on new-generation AEDs’ use during pregnancy and their association with ADHD in children.
METHODS
Inclusion and exclusion criteria
We used the five-stage approach proposed by Arksey and O’Malley.26 We followed the scoping review’s reporting guidelines in accordance with recommendations from the Preferred Reporting Items for Systematic Reviews and Meta-Analyses extension for Scoping Reviews (PRISMA-ScR).27 Studies were included if the authors examined pregnant women who were treated with new-generation antiepileptic and/or antiseizure drugs marketed after 1993 (e.g., lamotrigine, gabapentin, levetiracetam, oxcarbazepine, topiramate, vigabatrin, lacosamide, perampanel, brivaracetam, and eslicarbazepine). For comparison/reference group, we included all comparator types (i.e., studies with no treatment as comparator, an active comparator group, or exposed women with and without epilepsy). We included original studies that reported any of the following as the primary or the secondary outcome: ADHD, attention deficit disorder with hyperactivity (ADDH), conduct disorder, hyperkinesis, or hyperkinetic disorder (HKD). A detailed eligibility criteria is listed in Table 1.
TABLE 1. Inclusion and Exclusion Criteria.
| Inclusion criteria | Exclusion criteria | |
|---|---|---|
| Population(s) | Women who are:
|
N/A |
| Intervention(s) | New-generation AEDs, either in:
|
Old-generation AED (marketed before 1988) monotherapy |
| Comparison(s) | Women who are:
|
N/A |
| Outcome(s) | Diagnosis of at least one of the following:
|
Symptoms of, but no diagnosis of:
|
| Study designs |
|
|
| Date and language criteria |
|
|
Search strategy
A librarian developed the search strategy in collaboration with the team. Embase and Medline databases were searched in June 2019 for articles published in English from January 1988 to June 2019 (see Appendices 1 and 2 for the full Embase and Medline strategy, respectively). The literature search was updated on 28 April 2020 to locate recent reports, which identified 12 additional studies to be screened; however, none of these studies met our inclusion criteria. In addition, we supplemented the database searches with cited reference searching. Papers published in French with an English translation were considered for the review if available. Citations were managed using EndNote (version X9.3.3) and Rayyan (http://rayyan.qcri.org).
Study selection
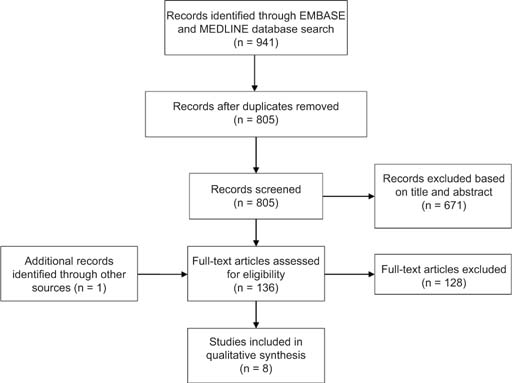
Two authors (CV and EC) independently screened titles and abstracts of the 805 studies identified. A total of 136 studies were selected as eligible for a full text review. All disagreements were resolved by consensus with a third reviewer (SE). See Figure 1 for the flowchart of study selection and screening.
FIG 1. PRISMA flow diagram of our scoping review examining new-generation AED use during pregnancy and the development of ADHD in offspring.
Data extraction
To chart our data, two team members (CV and EC) independently extracted information from eligible publications using a data extraction tool, followed by revision by a third member (AS). The tool provided detailed instructions for data extraction and charting.28 The following information was extracted: study characteristics (year of publication, study design, country, data source, and follow-up period), characteristics of the study participants and intervention type (number of participants, AED exposure, percentage of children with ADHD), and effect size (odds ratios, hazard ratios, P values, and confidence intervals). For the purposes of this review, AED exposure was organized into three categories: monotherapy (n = 7), polytherapy (n = 3), and exposure not specified (i.e., any AED) (n = 2).
RESULTS
Our search on Embase and Medline yielded 941 articles. Using the Bramer method,29 136 duplicates were removed using EndNote, leaving 805 articles to screen. Out of the 805 screened articles, a total of eight publications met our inclusion criteria (Figure 1).7,25,30–35 Of the eight studies, seven were cohort studies, and one was a meta-analysis. We stratified our results by AED monotherapy, polytherapy, and studies where AED exposure was not indicated/any AED exposure.
Monotherapy
The relationship between new-generation AED monotherapy and ADHD has been described in six cohort studies and one meta-analysis (Table 2), representing the largest available data in any of the exposure groups. Most studies were conducted in the United Kingdom and the United States (n = 5). The follow-up period of children in the included studies ranged from 4 years to 7 years and 11 months, or until ADHD diagnosis. Sample sizes of the AED-exposed group ranged from two to 1383 women, with the majority of the AED exposed group consisting of lamotrigine monotherapy (81%; 1779/2185). The sample size in the control groups ranged from four to 899,941 women, with three studies defining control as women without epilepsy and not on an AED, and two studies using a definition of women with epilepsy not exposed to any AED. None of the lamotrigine comparisons reached statistical significance for an increased risk of ADHD, with one study reporting an effect size of OR = 1.63 [95% CI: 0.41, 6.06]. One study reported a significantly decreased risk of ADHD among levetiracetam users compared to valproate monotherapy users, with an unstandardized coefficient (standard error) of −13.2 (6.0), (95% CI: −25.1 to −1.3, P-value = 0.030).34
TABLE 2. Studies Examining the Association between Antiepileptic Drug Monotherapy Use during Pregnancy and Attention-defect Hyperactivity Disorder in Offspring.
| Study Ref. | Design | Country | Data Source | Children followed until | AED User | Control | Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | n exposed | % ADHD | Definition | n | % ADHD | OR (aOR or cOR) |
HR (aHR or cHR) |
P-value and/or (95% CI) | |||||
| Cohort studies | |||||||||||||
| Bromley et al. (2013)25 | Cohort Study | The United Kingdom | Liverpool and Manchester Neurodevelopment Group | 6 years old | VBT | N/R | 1 ADHD diagnosis | Women without epilepsy not on an AED | 214 | 0 | N/A | N/A | N/A |
| LTG | 30 | 0 | N/A | N/A | N/A | ||||||||
| Christensen et al. (2019)30 | Cohort study | Denmark | Danish National Prescription Registry Danish Psychiatric Central Research Register |
Until ADHD diagnosis, ADHD prescription purchased, or 2015 (at least 4 years old) | LTG | 1383 | 2.96 | Not exposed to AED | 899,941 | 3.19 | N/A | aHR = 0.84 | (0.59–1.19) |
| OXC | 372 | 6.72 | N/A | aHR = 1.10 | (0.72–1.67) | ||||||||
| Miškov et al. (2016)31 | Cohort study | Croatia | Sestre milosrdnice University Hospital Centre | LTG | 28 | 3.57 | Women without epilepsy not on an AED | 128 |
N/R |
N/A | N/A | N/A | |
| GBP | 2 | 0 | N/A | N/A | N/A | ||||||||
| OXC | 2 | 0 | N/A | N/A | N/A | ||||||||
| Charlton et al. (2017)32 | Cohort study | The United Kingdom | Clinical Practice Research Datalink (CPRD) | 6 years, 3 months old | LTG | 122 | 0 | Women without epilepsy not on an AED | 6048 | 9.59 | 01 | N/A | N/A |
| Cohen et al. (2013)33 | Cohort study | The United Kingdom, The United States | Neurodevelopmental Effects of Antiepileptic Drugs Safety Group | 6 years old | LTG | Parenta | 61 4.92/ 6.56b | N/R | N/R | N/R | N/A | N/A | N/A |
| Teacherc | 36 13.89/5.564 | N/R | N/R | N/R | N/A | N/A | N/A | ||||||
| Parent and teacherd | 31 12.90e |
N/R | N/R | N/R | N/A | N/A | 0.1977f (3.63–29.83) | ||||||
| Huber-Mollema et al. (2019)34 | Cohort study | The Netherlands | European Registry of Antiepileptic Drugs and Pregnancy (EURAP-NL) | 6 years old to 7 years and 11 months old | LTG |
88 | 5.7/1.5g 4.6/4.4h 11.5/5.9i 5.7/1.5j |
VPA exposed | N/R | 3.8/08 3.8/4.39 7.7/010 0/011 |
B(SE) = −6.2(5.1)k −4.7(4.2) |
N/A | 0.221 (−16.2 – 3.8) 0.266 (−12.9 –3.6)l |
| LEV | 30 | 7.1/08 3.6/09 3.6/4.210 7.1/4.211 |
B(SE) = −13.2(6.0)12 −11.7(5.1) |
N/A | 0.030 (−25.1 – (−1.3)) 0.022 (−21.7 – (−1.7))13 | ||||||||
| Meta-analysis | |||||||||||||
| Veroniki et al. (2017)7 | Network meta-analysis and systematic review | 5 | N/R | N/R | LTG | N/R | N/R |
Not exposed to AED | N/R | N/R | 1.63 | N/A | (0.41–6.06) |
N/R, not reported. N/A, non applicable. OR, odds ratio (cOR, crude & aOR, adjusted). HR, hazard ratio (cOR, crude & aOR, adjusted). AED, anti epileptic drug. VBT, vigabatrin. LTG, lamotrigine. OXC, oxcarbazepine. GBP, gabapentin. VPA, valproate. LEV, levetiracetam. CBZ, carbamazepine. TPM, topiramate. PHT, phenytoin. WWE, women with epilepsy.
aParent ratings, Behaviour Assessment System for Children (BASC) clinical scales for ADHD.
bCombined type ADHD/Inattentive ADHD subtype
cTeacher ratings, Behaviour Assessment System for Children (BASC) clinical scales for ADHD.
dParent and teacher combined ratings, Behaviour Assessment System for Children (BASC) clinical scales for ADHD.
eCombined ADHD and inattentive ADHD subtypes.
f7% test of percentage
gMother/father ratings, Child Behavior Checklist (CBCL) and the Social Emotional Questionnaire (SEV). ADHD total.
hMother/father ratings, Child Behavior Checklist (CBCL) and the Social Emotional Questionnaire (SEV). ADHD attention deficit subtype.
iMother/father ratings, Child Behavior Checklist (CBCL) and the Social Emotional Questionnaire (SEV. ADHD hyperactivity subtype.
jMother/father ratings, Child Behavior Checklist (CBCL) and the Social Emotional Questionnaire (SEV. ADHD impulsivity subtype.
kMultilevel regression analyses unstandardized coeffects (B) and standard error (SE). VPA reference group.
lADHD total/ADHD attention deficit subtype. VPA reference group.
Polytherapy
The association between new-generation AED polytherapy and ADHD has been described in two cohort studies and one meta-analysis (Table 3). Both cohort studies took place in Europe (the United Kingdom and Croatia). The maximum follow-up period of children in the included studies was 6 years. Various combinations of AEDs were included such as lamotrigine and valproate; topiramate and valproate; lamotrigine and carbamazepine; and topiramate, carbamazepine, and phenytoin (Table 3). However, of the studies that reported specific numbers for women exposed to AED polytherapy, the maximum number exposed was two. The sample size in the control groups ranged from 4 to 214 women. Both studies defined control groups as women without epilepsy not on an AED, and one study used an additional group definition of women with epilepsy not exposed to AED. One study reported that children born to women with epilepsy exposed to lamotrigine and valproate polytherapy were 10 folds more likely to be diagnosed with an ADHD disorder than women without epilepsy not on an AED, OR = 9.97 (95% CI 1.82–49.40).
TABLE 3. Studies Examining the Association between Anti-epileptic Drug Polytherapy Use during Pregnancy and Attention-defect Hyperactivity Disorder in offspring.
| Study Ref. | Design | Country | Data Source | Children followed until | AED User | Control | Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | n exposed | % ADHD | Definition | n | % ADHD | OR (aOR or cOR) |
HR (aHR or cHR) | P-value and/or (95% CI) | |||||
| Cohort studies | |||||||||||||
| Bromley et al. (2013)25 | Cohort Study | UK | Liverpool and Manchester Neurodevelopment Group | 6 years old | LTG + VPA | N/R | 2 ADHD diagnosis | Women without epilepsy not on an AED | 214 | 0 | 9.97 | N/A | (1.82–49.90) |
| Miškov et al. (2016)31 | Cohort study | Croatia | Sestre milosrdnice University Hospital Centre | N/R | TPM + VPA |
2 | 0 | Women without epilepsy not on an AED ------------- WWE not on an AED |
128 ---- 4 |
N/R ------ N/R |
N/A | N/A | N/A |
| TPM + CBZ + PHT |
1 | 0 | N/R | N/R | N/R | N/A | N/A | N/A | |||||
| LTG + CBZ |
1 | 0 | N/R | N/R | N/R | N/A | N/A | N/A | |||||
| Meta-analysis | |||||||||||||
| Veroniki et al. (2017)7 | Systematic review and network meta-analysis | 5 | N/R | N/R | TPM + VPA |
N/R | N/R | Not exposed to AED | N/R | N/R | 1.51 | N/A | (0.00–46.48) |
N/R, not reported. N/A, non applicable. OR, odds ratio (cOR, crude & aOR, adjusted). HR, hazard ratio (cOR, crude & aOR, adjusted). AED, anti epileptic drug. VBT, vigabatrin. LTG, lamotrigine. OXC, oxcarbazepine. GBP, gabapentin. VPA, valproate. LEV, levetiracetam. CBZ, carbamazepine. TPM, topiramate. PHT, phenytoin. WWE, women with epilepsy.
Exposure not indicated/any AED exposure
Two cohort studies did not specify the AED therapy type of the exposed group (Table 4). Both studies took place in the United Kingdom, with a follow-up period of 6 years and 3 months or until study completion. Sample sizes of the AED-exposed group ranged from 43 to 546. The definitions and sample sizes of the control groups were not specified. In addition, none of the studies reported an effect size or percentage of ADHD among the controls.
TABLE 4. Studies Examining the Association between Anti-epileptic Drug use During Pregnancy and Attention-defect Hyperactivity Disorder in Offspring were the Specific AED Exposure was not Indicated.
| Study Ref. | Design | Country | Data Source | Children followed until | AED Users | Control | Effect | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Drug | n | % ADHD | Definition | n | % ADHD | OR (aOR or cOR) |
HR (aHR or cHR) |
P-value and/or (95% CI) | |||||
| Cohort studies | |||||||||||||
| Dean et al. (2002)35 | Cohort study | Scotland | Aberdeen Maternity Hospital | Until study ended | Any AED therapy | 261 | 1.15 | N/R | N/R | N/R | N/A | N/A | N/A |
| Charlton et al. (2017)32 | Cohort study | The United Kingdom | Clinical Practice Research Datalink (CPRD) |
6 years, 3 months old |
Any AED therapy | 546 | 6.04 | N/R | N/R | N/R | N/A | N/A | N/A |
| Any monotherapy not including VPA, CBZ, or LTG | 43 | 0 | N/R | N/R | N/R | N/A | N/A | N/A | |||||
N/R, not reported. N/A, non applicable. OR, odds ratio (cOR, crude & aOR, adjusted). HR, hazard ratio (cOR, crude & aOR, adjusted). AED, anti-epileptic drug. VBT, vigabatrin. LTG, lamotrigine. OXC, oxcarbazepine. GBP, gabapentin. VPA, valproate. LEV, levetiracetam. CBZ, carbamazepine. TPM, topiramate. PHT, phenytoin. WWE, women with epilepsy.
DISCUSSION
We examined the scope of the current published literature on the risk of ADHD after maternal exposure to new-generation AEDs during pregnancy. Our findings show that there is a relatively small but growing body of literature examining a diverse range of new-generation AEDs on the risk of ADHD, with most studies concluding no significant risk of ADHD among the offspring. The largest body of published evidence among new-generation AEDs was on lamotrigine monotherapy (n = 6), with an included meta-analysis reporting no significant increased risk (aOR of 1.63 [95% CI 0.41–6.06]).7 Nonetheless, lamotrigine and valproate polytherapy were shown to considerably increase the risk of ADHD with an aOR of 9.97 (95% CI 1.82–49.90).25 However, the effect observed in this study is most probably attributed to valproate and not lamotrigine, as the authors report significant effect with valproate monotherapy in the same study with an aOR of 6.05 (95% CI 1.65–24.53).25 Among the striking observations of this review, we noticed that some studies did not specify AED exposure type (n = 2), the number exposed to AED therapy (n = 1), the number in the control group (n = 3), and the effect size (n = 4). Future research examining the risk of ADHD after maternal exposure to new-generation AEDs would benefit from standardized and complete reporting of data.
Lamotrigine monotherapy was the most examined new-generation AED during pregnancy. Data from the meta-analysis show some evidence of a nonsignificant trend in the risk of ADHD, aOR of 1.63 (95% CI 0.41–6.06)7; however, the power might not be sufficient to detect a significant effect. However, the most recent and largest study to date—not included in the meta-analysis—examined 1383 exposed pregnancies and showed no significant effect (aHR = 0.84 [95% CI 0.59–1.19]).30 The majority of the studies in this review examining lamotrigine or monotherapy (five of the six studies) all had less than 100 women exposed to AED. We believe larger studies are needed to evaluate statistical merit of these findings. One possible way is to leverage observational studies using real-world data sources, which can increase statistical power, enhance the external validity, and broadly assess patient’s safety and clinical patterns to adjust for confounding factors.36
Frequently, patients might be prescribed AED polytherapy. Reasons include severe epilepsy, multiple seizure types that require drugs with different mechanisms of action, and in some other cases, synergistic beneficial effects are needed without the additive toxicity.37 We observed a shortage of evidence on the safety of AED polytherapy and the risk of ADHD in children. Besides the lack of well-conducted original studies, most of the reported comparisons included women exposed to valproate plus a new-generation AED. Given the established evidence of ADHD risk associated with valproate exposure, those comparisons assume minor additional value and have limited capacity to identify the true risk attributed to new-generation AED exposure.
A key finding from this review was the limited number of studies that addressed confounding by indication. When studying the risk of offspring ADHD, it may be impossible to distinguish between the risk attributed to the use of AEDs and the risk of the condition that triggered the use of the AEDs (i.e., epilepsy). As a consequence, women exposed and not exposed to AEDs might not be comparable, hampering valid causal inference.38 However, to ensure accurate and evidence-based conclusions, it is imperative to separate the effect of the epilepsy from the AEDs themselves. One way to address confounding by indication is using the right control group, particularly in a comparative safety study design. To identify if new-generation AEDs increase the risk of ADHD, studies should only compare AED users (e.g., users of lamotrigine to the users of gabapentin). This would enable us to control for the effect of epilepsy and solely examine the effect of AEDs. A second level of an appropriate comparison will be between a control group of women with epilepsy who do not use AED to women that do not have epilepsy (or to the general population). This will provide information on whether epilepsy per se (without the use of AEDs) plays a role in the risk of ADHD in the offspring. However, a limitation of this study is its inability to control for the severity level of epilepsy.
Strengths and limitations
The current scoping review has limitations and strengths that should be noted. First, it is possible that some relevant articles were missed, as only articles published in English were included. We did not provide a critical assessment of the quality of the evidence as this is a developing area of research.
However, our search strategy was comprehensive and was guided by a librarian. Second, we focused on a specific neurodevelopmental outcome, primarily ADHD in the offspring (ADHD, ADDH, conduct disorder, hyperkinesis, and HKD). A previous systematic review and meta-analyses used a broad definition of neurodevelopmental outcomes that included autism/dyspraxia, ADHD, language delay, neonatal seizures, psychomotor developmental delay, and social impairment.7 Using a broad definition would have been difficult to assess in a scoping review. Third, many of the included studies had small sample sizes, with some even collecting data from one patient. This repeated observation of incomplete reporting made it hard to draw informative conclusions.
Methodological limitations of several of these studies impact their validity, and the teratogenic effects of new-generation AEDs are still unknown. This review did not include animal studies, even though these studies may have had larger sample sizes and a more rigorous study design because this study’s objective was specific, focusing on the safety of AEDs on ADHD alone, and the extrapolation from animal model studies to humans is difficult.39
Lastly, there are many new-generation AEDs on the market. However, the studies only investigated six new-generation AEDs (gabapentin, lamotrigine, levetiracetam, oxcarbazepine, topiramate, and vigabatrin); hence, information about other new-generation AEDs is still unknown and highly needed.
CONCLUSIONS
Lamotrigine monotherapy holds the largest body of evidence among new-generation AEDs, concluding no significant risk of ADHD among the offspring. However, the current available evidence is scarce, with several methodological limitations in the published studies. Extricating the effect of AEDs from epilepsy itself and examining polytherapies that include new- and old-generation AEDs are challenges and areas that merit additional investigation. Further comparative safety studies with longer follow-up periods and larger sample sizes are needed to accurately quantify the true impact of exposure to new-generation AEDs during pregnancy and ADHD.