Case Report
Hemorragic presentation of Listeria Monocytogenes rhombencephalic abscess
Paola Feraco1,2, Francesca Incandela3, Flavia Stallone3, Francesca Alaimo3, Laura Geraci3, Francesco Bencivinni3, Giuseppe La Tona3 and Cesare Gagliardo3
1Department of Neuroradiology, Ospedale S. Chiara, Azienda Provinciale per i Servizi Sanitari, Trento Italy
2Department of Experimental, Diagnostic and Specialty Medicine (DIMES), University of Bologna, Bologna, Italy
3Department of Biomedicine, Neuroscience and Advanced Diagnostic - University of Palermo, Palermo, Italy
Corresponding author: paola.feraco@apss.tn.itSubmitted: 18 may 2020. Accepted: 08 june 2020. Published: 16 July 2020.DOI: 10.15586/jptcp.v27i3.704
ABSTRACT
Listeria monocytogenes (LM) bacterium is a cause of central nervous system (CNS) infection and the most common cause of rhombencephalitis in immunocompetent elderly.
A prompt identification of this condition should be always desirable, since its clinical manifestations are often unspecific with prodromal symptoms leading to high rates of morbidity and mortality if underestimated.
CNS listeriosis magnetic resonance imaging (MRI) findings are generally not specific. However, in the appropriate clinical setting, focal brainstem hyperintensity on T2-weighted pulse sequences associated with ring-enhancement pattern after i.v. contrast media injection should be suspicious of LM abscess. The diagnosis cannot exempt from anamnestic-clinical-investigation data correlation to exclude mimicking.
We report the case of a 72-year-old man with fever, headache, vomiting, and persistent hiccups with an increasing walking difficulty. A progressive worsening of the state of consciousness led him to a stupor state. Brain MRI examination detected multiple rhombencephalic abscesses. Among these, one was with atypical hemorrhagic presentation. The presence of hemorrhage, uncommon for listeria abscesses, may further complicate their detection, with consequent delayed treatment. The diagnostic hypothesis was confirmed by cerebrospinal fluid examination, which was confident with LM infection. Clinical and neuroradiological state improved after antibiotic therapy.
Keywords: Listeria monocytogenes, brain magnetic resonance imaging, MRI, hemorrhage, infectious disease
INTRODUCTION
Listeria monocytogenes (LM) bacterium is a cause of central nervous system (CNS) infection, due to hematogenous spread from the gastrointestinal tract. It could lead to a high rate of morbidity and mortality, especially in consideration of the unspecific prodromal symptoms (fever, dizziness, nausea, vomiting) and more rarely signs of meningeal irritation.1,2
The most common CNS manifestation is represented by meningitis, since epithelium of the choroid plexus enables LM to gain access to CNS. However, less commonly, it may reach the brain parenchyma via the cerebral capillary endothelium resulting in cerebritis and rhombencephalitis which may lead to brain abscess formation.1
This occurs especially in immunosuppressed patients, infants, and elderly people,1 although brainstem and cerebellum infections have been reported in previously healthy adults too.2
Even though CNS listeriosis magnetic resonance imaging (MRI) findings are generally unspecific, in the appropriate clinical setting, focal brainstem hyperintensity on T2-weighted pulse sequences, with ring-enhancing mass and significant perilesional edema, should suggest the possibility of LM abscess.1 We report the case of an elderly man with LM rhombencephalic abscesses, showing atypical hemorrhagic presentation, emphasizing neuroradiological differential diagnosis.
CASE REPORT
A 72-year-old man, recently treated for cardiac arrhythmia, was hospitalized for fever (41.4°C), headache, vomiting, and persistent hiccups. Neurological examination showed asthenia in the lower limbs with increasing walking difficulty. No signs of meningeal irritation were detected. The progressive worsening of the state of consciousness led to a soporous state (GCS 4). Hence, an orotracheal intubation was performed.
Blood counts revealed neutrophilic leukocytosis (WBC 16860, N 85.8%, L 3.6%, M 10.5%), with normal flogosis index (CRP 6.56 mg/dl). The CSF was clear with “rock water” appearance, characterized by a high protein concentration (1,518 mg/L), glucose concentration of 48 mg/dl, and high LDH concentration of 75 U/L.
Empirical antimicrobial treatments with piperacillin-tazobactam, ceftriaxone, vancomycin, and acyclovir were started. Subsequently, PCR microbiological examination of the CSF revealed LM positivity, and ampicillin/sulbactam and gentamicin therapy was started.
Nevertheless, an increase of laboratory index (WBC 20,590 and CRP 25,065 mg/dl) was recorded with a subsequent worsening of patient’s clinical presentation: he was sleepy, showing left upper limb hyposthenia. A brain MRI examination revealed small oval lesions in the pons, bulb, and left middle cerebellar peduncle, showing an iso-hypointense core on T1-weighted sequences with an iso-hyperintense irregular ring, iso-hypointense signal on T2-weighted sequences with a hyperintense ring. Among these, the larger lesion showed blooming artifacts on T2*-weighted gradient-echo sequence, no significant restriction of water molecules on apparent diffusion coefficient (ADC) maps, and significant perilesional edema (Figure 1). T1-weighted sequences acquired after i.v. contrast medium administration revealed typical ring-enhancement pattern. The neuroradiological findings of the bigger lesion were consistent with intra-lesional hemorrhage (mixed oxy- and deoxy-hemoglobin related to hyperacute/acute hemorrhage. Although blooming artifacts on T2* are usually consistent with hemosiderin, in our case paramagnetic substances (free radicals from phagocytosis) cannot be excluded. Laboratory investigations supported LM abscess diagnosis. Antibiotic therapy was reset on a large spectrum, with a significant improvement of clinical and neuroradiological setting. In particular, reduction of the larger lesion’s size with disappearance of the ring-enhanced wall after 25 days was consistent with signs of therapy response with only a small dot of enhancement after i.v. contrast media administration (Figure 2).
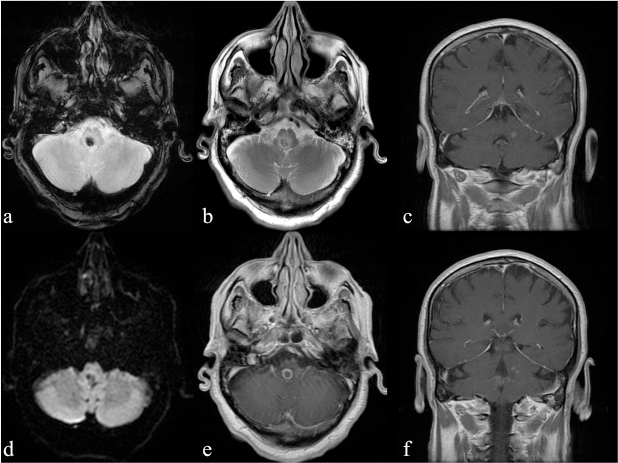
FIG 1. (a-f) Magnetic resonance imaging (MRI) at admission. Rhombencephalic, bulbar lesion shows blooming artefacts on T2*-weighted sequence (a) and no restriction in DWI (c) consistent with haemorrhage at late-acute stage; (b) T2-weighted images, shows significant perilesional oedema while T1-weighted post-contrast images (e) show ring-enhancement appearance of the bulbar lesion (arrow) and other small enhancing dots on the coronal views (c,f) consistent with multiple Listeria Monocytogenes lesions.
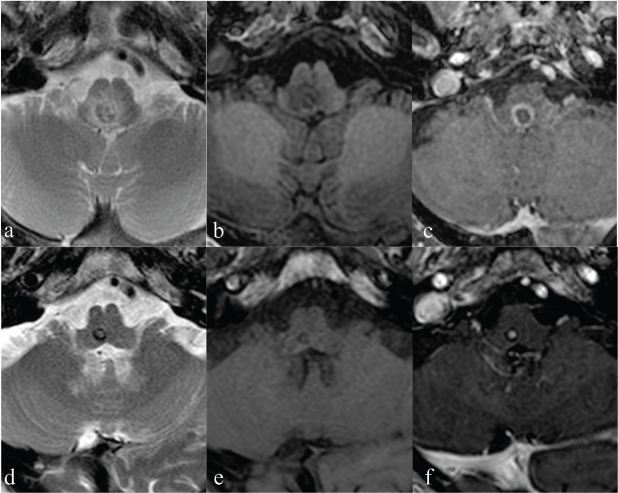
FIG 2. (a-c) MRI at admission: detail of the bigger lesion showing a iso-hypointense core on T2-weighted sequence consistent with diamagnetic blood products (oxyhaemoglobin) and an hyperintense ring consistent with paramagnetic blood products (deoxyhaemoglobin) (a). Lesion core is iso-hyperintense on T1-weighted sequences surrounded by an hyperintense irregular ring (b). I.v. administration of paramagnetic contrast media demonstrates the typical ring enhancement of the abscess (c). (d-f) Follow-up MRI exam performed 25 days later: detail of the bigger lesion that is now smaller in size and presents signs of haemorrhage at late subacutestage: hyperintense in the core at T2-weighted images and iso- hyperintense in T1-weighted. Moreover, it was reduced in size with disappeared enhancing of wall on T1-weighted sequences acquired post-contrast injection (d), while a residual small enhanced dot is evident in the center of the lesion consistent with the residual infection.
DISCUSSION
LM brain abscesses, although reported in literature.1 are extremely rare, accounting for approximately 1–10% of CNS listerial infections.1,2 In particular in a recent systematic prospective study, which allows an unbiased description of neuroradiological unspecific features of listeriosis, LM abscesses was defined as “nodules or masses with peripheral enhancement after injection of gadolinium chelates on T1-weighted images on MRI,” with “hyperintensity on MRI diffusion weighting images” (DWI),3 accounting for 6% of listeriosis neurological features.
In our case, MRI scan revealed also nodular lesions, characterized by T1 hypointensity, with T2/FLAIR hyperintensity and “target” appearance on T1 post-contrast sequences. The ring enhancement of abscess, detected on MRI, represents the wall of the abscess provided by granulation tissue, which usually develops at the “late capsule formation stage” (14 days and beyond),4 appearing hypointense on T2-weighted sequences, but it represents also an interstitial enhancement for vascular permeability increasing due to inflammation;5 while the central non-enhancing portion, hypointense on T1-weighted sequences, is due to pus.
However, these neuroradiological findings of Listeria brain abscesses are similar to other ring-enhancing lesions, since they may also mimic primary or metastatic brain tumors, in particular high-grade necrotic cavitated tumors (e.g., glioblastoma multiforme).6,7 Thereby neuroradiological differential diagnosis sometimes can be difficult. In particular, in our case, the differential diagnosis of multiple rhombencephalic lesions with different appearance included metastases, but also CLIPPERS (chronic lymphocytic inflammation with pontine perivascular enhancement responsive to steroids), which represents a rare CNS inflammatory disorder involving predominantly the pons as well as additional parts of the rhombencephalon.8
Usually, in these cases, a combination of sequences, including DWI (with related ADC maps), perfusion-weighted imaging (PWI), and MR spectroscopy (MRS), although they were not all obtained in our case, may allow to distinguish abscess from other brain lesions.
In particular, increased signal on DWI (caused by the high cellularity and viscosity of pus) with corresponding reduced ADC values usually helps to distinguish abscess from necrotic or cystic tumors, which typically exhibit reduced DWI signal and normal or increased ADC.9 However, metastatic hypercellular ring-enhancing brain lesions have been shown to demonstrate restricted diffusion.10 Furthermore, intracellular oxyhemoglobin (as seen in hyperacute hemorrhage) displays high signal intensity on DWI and is characterized by ADC values lower than those of normal brain tissue, similar to brain abscess (11), making DWI restriction sensitive but not specific for brain abscess diagnosis.
In our case, the DWI restriction and reduced ADC values were detected only in two small lesions, whereas the larger ring-enhanced lesion displayed low signal intensity in DWI due to the presence of the magnetic field inhomogeneity caused by paramagnetic intracellular deoxyhemoglobin in acute hematoma.11 Indeed, the presence of hypointense brain stem foci notably at T2*-weighted gradient-echo images (GRE), as in our case, suggests the presence of hemorrhage inside the larger abscess. This hypothesis was confirmed by the follow-up MR exam. Indeed, after therapy administration, the ring-enhancement disappeared (due to therapy response) but the signs of hemorrhage on GRE T2*-weighted images were unchanged. Hence, the presence of hemorrhage in the setting of a ring-enhanced lesion could further complicate the diagnosis. Indeed, both primary and secondary CNS tumors may show signs of hemorrhage.
Moreover, multiple T2*-weighted hypointense areas, some corresponding to contrast-enhancing lesions, were also reported in CLIPPERS but they were related to iron deposition secondary to chronic inflammation, without the sign of bleeding.8
On the other hand, another confounding factor, which could have complicated the diagnosis, was the patient’s atrial fibrillation treated before hospitalization. Indeed, infective endocarditis is one of the most common cause complicating this kind of cardiac treatment, and a correlation with cerebral microbleeds (detected on brain MRI as round T2* hypointensities) has been described although they typically show supratentorial localizations.12
Hemorrhagic pyogenic abscesses have been well described.13 The pathophysiology may involve destruction of neoangiogenic fragile blood vessels of the abscess wall, alternatively thrombosis of the surrounding vessels due to increasing abscess mass effect, or eventually a direct free radicals damage leading to abscess bleeding.13 However, usually, hemorrhagic components are located in the abscess’s wall, while in our case the lesion was completely hemorrhagic even if the presence of paramagnetic substances (free radicals from phagocytosis) cannot be excluded.
Since PWI provides information about the vascularization of a lesion, it may result helpful for differential diagnosis of ring-enhancing lesions. Typical PWI pattern of a pyogenic brain abscess is characterized by a low relative cerebral blood volume (rCBV) in the enhancing capsule, whereas necrotic brain tumors (i.e., glioblastoma and metastasis) show elevated rCBV as a result of neoangiogenesis.10 Pseudotumoral demyelinating lesions that, however, rarely primarily involve the brain stem may also present a typical ring enhancement pattern with PWI findings similar to necrotic brain tumors. However, in the case of brain abscesses, PWI findings depend very much on the time in which the MRI examination is performed in relation to capsule organization. As a matter of facts, high rCBV values may be found in the early stage, while in the late stages rCBV values are typically low because of more and more fibroblasts within the capsule.
MRS is just as helpful in the differential diagnosis of ring-enhancing lesions, and in particular, it may be useful to differentiate brain abscess from necrotic tumors.14 As a matter of facts, abscesses lack typical peaks that characterize normal brain tissue (N-acetyl-aspartate, choline, and creatine) in favor of pathological peaks such as lipids, lactate, and amino acids. On the other hand, other ring-enhancing lesions as tumefactive demyelinating lesions and subacute infarcts typically present brain typical metabolite peaks.
Lastly, it must be said that in a clinical scenario, it can be difficult to apply advanced MRI techniques to small lesions located in difficult locations. Thus, it is difficult, if not impossible, to base the diagnosis on a single MRI advanced technique. Multiparametric MRI, however useful, requires that all the acquired pulse sequences must be considered to pose the most plausible diagnostic hypothesis. Especially if one does not consider the clinical and laboratory data, which should be considered always mandatory, it would be easy to fall into error.
Recently, a perspective study3 reported a hemorrhage rate of 15% in patients suffering from LM infection. In particular, bleeding characterized from petechiae, hematoma, subarachnoid, or ventricular hemorrhage was reported, but not within abscesses, while, to date, only a small report15 described this particular finding.
The presence of hemorrhage could reflect Listeria endothelial tropism, since LM might invade endothelial cells, either directly or via infected monocytes16. Moreover, a bacterial invasion, with localized inflammation and necrosis of brain parenchyma and surrounding blood vessels, leading to exudation of blood products, has been proposed15. Thus, clinical and anamnestic data are essential. Supportive findings (like positive blood culture or presence of septic focus) help to rule out other differential diagnosis.
CONCLUSION
Neuro-listeriosis is not associated with any pathognomonic neuroradiological sign or any specific combination of signs, showing many potential mimicking. Hemorrhage is rare but could occur, leading to complex brain abscess neuroradiologic appearance, which may delay diagnosis and treatments. Moreover, a strict MRI follow-up is essential to confirm the diagnosis and the therapy response.
CONFLICTS OF INTEREST
The authors declare that there is no conflict of interest.
FUNDING
The authors received no funding.
DATA AVAILABILITY STATEMENT
No datasets were generated from this case report.
COMPLIANCE WITH ETHICAL STANDARDS
No ethics approval needed for this case report.
REFERENCES
- Arslan F, Ertan G, Emecen AN, Fillatre P, Mert A, Vahaboglu H. Clinical presentation and cranial MRI findings of Listeria monocytogenes encephalitis: A literature review of case series. Neurologist. 2018;23:198–203. https://doi.org/10.1097/NRL.0000000000000212
- Kayaaslan BU, Akinci E, Bilen S, et al. Listerial rhombencephalitis in an immunocompetent young adult. Int J Infect Dis. 2009;13(2):65–7. https://doi.org/10.1016/j.ijid.2008.06.026
- Charlier C, Poirée S, Delavaud C, et al. Imaging of human neurolisteriosis: A prospective study of 71 cases. Clin Infect Dis. 2018;67(9):1419–26. https://doi.org/10.1093/cid/ciy449
- Britt RH, Enzmann DR, Yeager AS. Neuropathological and computerized tomographic findings in experimental brain abscess. J Neurosurg. 1981;55:590–603. https://doi.org/10.3171/jns.1981.55.4.0590
- Schwartz KM, Erickson BJ, Lucchinetti C. Pattern of T2 hypointensity associated with ring-enhancing brain lesions can help to differentiate pathology. Neuroradiology. 2006;48:143–9. https://doi.org/10.1007/s00234-005-0024-5
- Chawla S, Wang S, Mohan S, et al. Differentiation of brain infection from necrotic glioblastoma using combined analysis of diffusion and perfusion MRI. J Magn Reson Imaging. 2019;49:184–94. https://doi.org/10.1002/jmri.26053
- Smirniotopoulos Murphy FM, Rushing EJ, Rees JH, Schroeder JW. Patterns of contrast enhancement in the brain and meninges. RadioGraphics. 2007;27(2):525–51. https://doi.org/10.1148/rg.272065155
- Pesaresi I, Sabato M, Desideri I, Puglioli M, Moretti P, Cosottini M. 3.0T MR investigation of CLIPPERS: Role of susceptibility weighted and perfusion weighted imaging. Magn Reson Imaging. 2013;31:1640–2. https://doi.org/10.1016/j.mri.2013.06.012
- Mortimer A, O’Leary S, Bradley M, Renowden SA. Pitfalls in the discrimination of cerebral abscess from tumour using diffusion-weighted MRI. Clin Radiol. 2010;65:488–92. https://doi.org/10.1016/j.crad.2009.12.012
- Muccio CF, Esposito G, Bartolini A, Cerase A. Cerebral abscess and necrotic cerebral tumours: Differential diagnosis by perfusion-weighted magnetic resonance imaging. Radiol Med. 2008;113:747–57. https://doi.org/10.1007/s11547-008-0254-9
- Kang BK, Na DG, Ryoo JW, Byun HS, Roh HG, Pyeun YS. Diffusion-weighted MR imaging of intracerebral hemorrhage. Korean J Radiol. 2001;2(4):183–91. https://doi.org/10.3348/kjr.2001.2.4.183
- Hess A, Klein I, Iung B, et al. Brain MRI findings in neurologically asymptomatic patients with infective endocarditis. Am J Neuroradiol. 2013;34:1579–84. https://doi.org/10.3174/ajnr.A3582
- Gupta RK, Tomar V, Awasthi R, et al. T2*-weighted MR angiography substantially increases the detection of hemorrhage in the wall of brain abscess: Implications in clinical interpretation. Neuroradiology. 2012;54:565–72. https://doi.org/10.1007/s00234-011-0952-1
- Chiang IC, Hsieh TJ, Chiu ML, et al. Distinction between pyogenic brain abscess and necrotic brain tumour using 3-tesla MR spectroscopy, diffusion and perfusion imaging. Br J Radiol. 2009 Oct;82:813–20. https://doi.org/10.1259/bjr/90100265
- Nham B, Baskin J, Choong H. Listeria rhomboencephalomyelitis complicated by hemorrhagic transformation. Neurology. 2017;89:872–3. https://doi.org/10.1212/WNL.0000000000004273
- Wilson SL, Drevets DA. Listeria monocytogenes infection and activation of human brain microvascular endothelial cells. J Infect Dis. 1998;178:1658–66. https://doi.org/10.1086/314490

