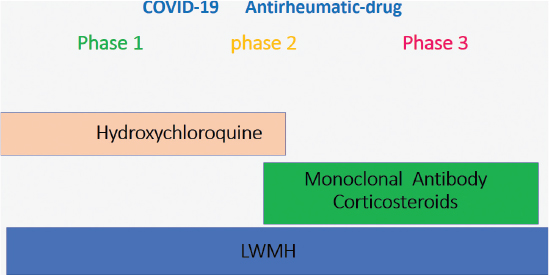
FIG 1. The three phases of coronavirus disease and the drugs employed at each stage.
Review
Marco Valentini1 and Hassan Zmerly2,3
1Rheumatology Unit, San Pier Damiano Hospital, Faenza (RA), Italy
2Orthopaedic Unit, San Pier Damiano Hospital, Faenza (RA), Italy
3UCM Malta – Ludes Lugano Campus, Lugano, Switzerland
COVID-19 disease is the most recent pandemic, since it has affected more than four and a half million people and caused more than 300,000 deaths. It is a very complex systemic disease in terms of pathogenesis, treatment, and prognosis. Pharmacological treatment may include antiviral and antimalarial drugs, antibiotics, monoclonal antibodies, corticosteroids as well as low-molecular-weight heparins to prevent the evolution of the disease from reaching the severe inflammatory phase that can lead to respiratory complications, multiple organ failure, disseminated intravascular coagulation (DIC), and finally death. Therefore, pending the development of the much sought-after vaccine, there needs to be a multidisciplinary approach to tackling this disease, and it is essential to use different medical treatments at the correct pathogenic moment. The aim of this article is to evaluate the rationale and reason behind the use of antirheumatic drugs, by expert point of view, in the various phases of the disease. Another important aspect in the management of the disease is to identify patients at high risk, both to change their lifestyle and to correct the state of their health through non-pharmacological measures for improving their immuno-balance. Our literature review reveals the important role and the therapeutic potential of antirheumatic agents in preventing the progression of the disease and aiding recovery from the disease. However, there is a lack of clinical evidence to support the use of these agents, indicating that further randomized controlled studies are required.
Keywords: COVID-19, phases, treatment, antirheumatic drugs
Coronaviruses were identified in the 1960s. They are positive-stranded RNA viruses, and the name “crowns” is due to their appearance as seen under an electron microscope. They can infect humans and certain animals (birds and mammals). Common coronaviruses (beta coronavirus and alpha coronavirus) can cause infections ranging from common colds to severe respiratory problems. These disorders are mainly due to beta coronaviruses: SARS-CoV, Mers-Cov.1–3 In December 2019, the first cases of infection caused by a new human coronavirus were reported in the Wuhan region in China. In the first half of February, the International Committee on Taxonomy of Viruses (ICTV) assigned a definitive name to the new coronavirus: “Severe Acute Respiratory Syndrome coronavirus 2” (SARS-CoV-2). The name was assigned by a group of experts in charge of studying the new coronavirus strain. According to this pool of scientists, the new coronavirus is the “brother” of the virus that caused SARS (SARS-CoV), hence the name SARS-CoV-2.
This infection took on the characteristics of a pandemic, which was officially declared by the WHO in March 2020. Based on literature date, pharmacological treatment of COVID-19 may include antiviral and antimalarial drugs, antibiotics, monoclonal antibodies, corticosteroids, and low-molecular-weight heparins (LMWH) to prevent the evolution of the disease from reaching the severe inflammatory phase that can lead to respiratory complications, multiple organ failure, disseminated intravascular coagulation (DIC), and finally death. The aim of our work is to evaluate the rationale and reason behind the use of antirheumatic drugs in the various phases of the disease, and to identify patients at high risk, both to change their lifestyle and to correct the state of their health through non-pharmacological measures for improving their immuno-balance.
We performed a literature review in PubMed/MEDLINE to evaluate the rationale and reason behind the use of antirheumatic drugs in COVID-19 treatment. Articles about the COVID-19 phases and the role of antirheumatic drugs for COVID-19 treatment were taken into consideration.
COVID-19 can be divided into three phases with different pathogenesis, treatment, and prognosis. Antirheumatic drug, with antiviral, antibiotic, and LMWH (Figure 1), could play an important role in the treatment of COVID-19 to avoid complications that require invasive ventilation and the progression to the death. Antirheumatic drugs are important in the prevention of infection and the treatment of all disease phases. The results are summarized in Table 1.

FIG 1. The three phases of coronavirus disease and the drugs employed at each stage.
COVID-19 can be distinguished into three progressive phases4–7: (1) The first phase is a viral phase characterized by fever, fatigue, dry cough, anosmia, taste alteration, nasal congestion, and diarrhea. These symptoms are generally mild and can simulate a flu-like viral infection. In this condition, an initial lymphopenia and an increase in C-reactive protein (CRP) and PT-INR were observed; this is a typical expression of IL-65. (2) The second phase is a pulmonary phase characterized by dyspnea and hypoxia. (3) The third phase is characterized by respiratory distress syndrome (SARS) that can evolve into a clinical manifestation of vasculitis with embolism, DIC, septic shock, and metabolic acidosis.7
The three phases are characterized by a distinct pathogenesis. In the first phase, the immune response is typically that of a viral respiratory syndrome; the mechanisms are similar to those of a flu syndrome. In fact, the Italian Study in Vo Euganeo6 found that a non-eligible number of patients (an estimated 43% of asymptomatic or oligo symptomatic) faced and resolved this infection at home without reporting damage or evolution.
If the first phase is not completely resolved, the clinical manifestation evolves toward the second phase, which has been called the respiratory phase, in which the patient begins to experience dyspnea. At this stage, auscultation of the chest is not indicative, but there is a progressive and rapid decrease in oxygen saturation. In this phase, pulmonary CT images show interstitial pneumonia with typical “ground-glass opacity” and “consolidation” aspects even in patients with mild or minimal respiratory symptoms.8
From the second phase, the disease can evolve toward the third that of respiratory distress syndrome with possible evolution toward a DIC, multisystem organ failure, and death. Laboratory examinations show extremely high concentrations of interleukin 1 (IL-1), interleukin 6 (IL-6), D-dimer, ferritin, troponin, and BNP (B-type natriuretic peptide).9 The cytokine storm is predominated by an elevated level of IL-6, which is a typical expression of an excess of Th17 lymphocytes.10–12
Moreover, gender seems to be an important factor in the manifestation of the disease, where females generally exhibit more benign symptoms than males.13 Several hypotheses have been developed; however, the most recognized one is that females express fewer COVID-19 receptors on their cell surface than males. Therefore, a first consideration is to identify all the risk categories of this immune imbalance.
In rheumatology/orthopedics, patients with uncontrolled rheumatoid arthritis (RA) show Th17 and Th1 hyperactivation, demonstrated by the persistent high level of CRP. A similar alteration is seen in psoriatic arthritis (PsA) in the polyarticular form. In the case of systemic lupus erythematosus (SLE), the problem is different; in this disease, there is Th2 alteration, which is expressed as leukopenia and altered plasma-cell response, responsible for a greater susceptibility to viral infections. Dysmetabolic inflammation typical of diabetic patients with metabolic syndrome also produces a high constant C-reactive protein; metabolic inflammation is frequently associated with chronic inflammatory rheumatism. Another consideration in assessing the population at risk is the presence of low blood levels of 25 OH D14–16; it is known that levels above the normal range of 25 OH D determine an increase in T Reg and a relative decrease in Th 17. Isaia et al.15 have shown that in Italian females over 70 years old, there is a high incidence of hypovitaminosis D (25 OH D less than 16 mg/ml) of about 76%.
Chloroquine and hydroxychloroquine have been used since 1940 in the treatment of autoimmune inflammatory rheumatisms.17,18 With regard to possible collateral effects, hydroxychloroquine presents a greater safety profile than chloroquine. Hydroxychloroquine and chloroquine are not associated with an increased risk of infectious complications or cancer.19 The most common adverse effects of these antimalarial drugs are gastrointestinal effects, including nausea, vomiting, diarrhea, and abdominal discomfort. In addition, several studies have reported the occurrence of hydroxychloroquine-associated myopathy and hydroxychloroquine-mediated and/or chloroquine-mediated cardiotoxic effects, including rhythm disorders (such as a prolonged QT interval) and the development of cardiomyopathy in patients with rheumatic diseases. However, the risk of antimalarial arrhythmia is increased in patients already suffering from heart rhythm disorder and it is dose-dependent.20
An increased risk of retinopathy has resulted in ophthalmology guidelines, recommending a maximum daily dose of 5.0 mg/kg actual body weight for hydroxychloroquine. Annual ophthalmology control is requested.19
First used in RA, hydroxychloroquine was recently found to be applicable in the therapy of connectivities (lupus erythematodes, undifferentiated connective tissue disease). The actions of such drugs on the immune system occur at multiple levels. At the level of lysosomes, these drugs increase the lysosomal pH and consequently inhibit the autophagy mechanisms that amplify the inflammatory response. At the level of the Toll-like receptor (TLR), they act by binding to the endosome and preventing the transcription of RNA and DNA, and consequently inhibit the transcription and production of pro-inflammatory cytokine (IL-6, TNF alfa, IL-1).21
In COVID-19 infection, chloroquine and hydroxychloroquine link to the glycoprotein E2 on the surface of the virus, preventing its liaison with the cell. This action could be an explanation for the reduction of viral load. To confirm this, Gautret22 has shown a 70% recovery healing rate for patients with SARS-CoV-2 in therapy with hydroxychloroquine 200 mg/TID, with a negative result from the polymerase chain reaction (PCR) on nasal swab. In cases of patients treated in association with azithromycin (500 mg on day one, followed by 250 mg per day for the next 4 days), 100% of patients tested negative for the PCR nasal swab test; however, the association of hydroxychloroquine or chloroquine and azithromycin in patients with COVID-19 is potentially linked to the risk of adverse events including arrhythmia and electrophysiological cardiac conditions of prolonged QT interval.23 Hydroxychloroquine also determines the decrease of the production of pro-inflammatory cytokine, in particular the IL-1 and the IL-6, preventing the cytokine storm that occurs in the evolution of the clinical disease in phase 2 or 3.24 On the basis of the above, the therapy with antimalarials should be placed at the very early stages of the disease (phase 1),25 in order to prevent the evolution toward the cytokine cascade that leads to the dramatic events of interstitial pneumonia and DIC.
Wengliu et al.26 have described how COVID-19, through glycoproteins Spike ORF 1AB, ORF 7, and ORF 8, could coordinate an attack on the heme group of the chain 1 beta hemoglobin to dissolve the iron in the form of porphyrin. This mechanism would reduce the availability of hemoglobin to transport O2 and CO2 and consequently result in the collapse of oxygen saturation. Hydroxychloroquine and chloroquine would prevent this attack on the heme group and consequently on the hemoglobin through the binding (liaison) of ORF to the heme to form porphyrins. Microcytic anemia could protect against disease. Accepting this mechanism, chloroquine and hydroxychloroquine would act with a typically antimalarial effect as well as an antiviral and cytokine modulator mechanism.26
The second and third phases of the disease are characterized by a rapid and exponential growth of IL-2 and IL-6, as well as gamma interferon, d-dimer, and BNP.11
The cytokine storm is responsible for the evolution of the symptoms of SARS-CoV-2 toward a dramatic manifestation of DIC.12 Patients who develop this critical state have concentrations of IL-10, 10 times higher than the control patients. The cytokine storm27 that starts from the second phase of the disease and then grows exponentially in the third phase determines a hemophagocytic lymphohistiocytosis (sHLH), which determines a systemic hyperinflammation with multi-organ involvement that can lead to death.12
Tocilizumab ® is a recombinant humanized monoclonal antibody of the IgG1 class, directed against the soluble IL-6 receptor (sIL-6R) and bound to the membrane (mIL-6R). It has been used for many years in the therapy of patients with RA refractory to DMARS and anti TNF alpha, and Horton arteritis.
Italian guidelines support the use of tocilizumab (at the dosage of 8 mg/kg, with a second dose after 12 h and a possible third dose after a further 24–36 h) according to clinical response.28
Current reports suggest a positive response from 70% of patients treated with this therapy. A main contraindication to this therapy applies if the patient has previously been infected with tuberculosis since this therapy can trigger a relapse of TB and evolve toward an aspect of miliary diffusion.
Administration of tocilizumab results in a rapid decrease in C-reactive protein, which is the serological marker of IL-6. In addition, rapid growth of blood oxygen pressure and dyspnea are reported, with a reduction in fever.29 Tocilizumab is indicated in phase 2 or 3 of the SARS-CoV-2 disease.10,29
Sarilumab is a monoclonal antibody to the IL-6 receptor. A study by the Italian drug agency is underway for this ongoing molecule of COVID-19. The rationale for use is the same as for tocilizumab.29
During hyperimmune inflammatory response, the first cytokine to rise is IL-1 followed by IL-6 and TNF alpha. Therefore, IL-1 should be modulated at an early stage of the immune response. Anakinra® is a receptor antagonist for IL-1 produced in Escherichia coli cells using the technology of recombinant DNA. It has been used in the past for the treatment of RA and now in the therapy of autoinflammatory diseases; the administration is daily and subcutaneous. Trials by the Italian drug agency are in progress.10 The association of anakinra with emapalumab, an anti-interferon gamma (anti-IFNγ) monoclonal antibody for phase 3 SARS-CoV-2 treatment, is under investigation in clinical trials.30
Canakinumab is a human anti-interleukin 1 beta (IL-1 beta) monoclonal antibody. It is currently authorized in Italy for the treatment of periodic autoinflammatory fever, Still’s disease, and refractory gouty arthritis.29
Jak inhibitors (tofacitinib, baricitinib) are molecules used in the therapy of refractory RA, which act at the cellular level by modulating the transcription of DNA; they modulate the transcription of extracellular messages to the nucleus, inhibiting the production of several pro-inflammatory cytokines.30 One of the mechanisms by which the virus enters the cell is endocytosis. This is regulated by ACE 2 receptors, principally AT2 alveolar epithelial cells. One of the regulators of endocytosis is the numb associated kinase (NAK) family, including AP2-associated protein kinase (AAK1) and another kinasi named GAK. Blocking these two kinasi may block endocytosis and the secondary assembly of virus particles.
Baricitinib is an oral ts-DMARD, which inhibits Janus-kinases 1 and 2. It has been identified as a potentially useful molecule in patients with COVID-19, for a dual action of mitigation of the inflammatory cascade and reduction of the entry of the virus into lung cells. A dose of 2 mg or 4 mg daily is able to inhibit AAK1 and GAK, reducing both the inflammation cascade and viral entry.31
Tofacitinib is an oral ts-DMARD Janus kinase inhibitor (JAK-inhibitor). In vitro studies appear to demonstrate that tofacitinib inhibits IL6/STAT3 signaling more significantly than baricitinib. It is currently approved in Italy for the treatment of RA and PsA.
For baricitinib and tofacitinib, there are studies that are approved or under evaluation. These drugs have been used in a few COVID-19 patients.31,32
Several trials are now in progress to evaluate the effectiveness of JAK inhibitors on controlling the evolution of the COVID-19 disease. If the preliminary data are confirmed, this drug should be placed in the second phase of COVID-19 disease.
The administration of an appropriate dosage of vitamin D could be placed in the context of an intervention to prevent and reduce risk factors toward unfavorable evolution of SARS-CoV-2.
The immunomodulatory role of vitamin D and its antagonistic effect on viral replication in the respiratory tract has long been known.33
Borella34 examined the interactions between vitamin D, the immune system and infectious diseases, highlighting the association between hypovitaminosis D and respiratory and enteric infections. Otitis media, Clostridium infections, vaginosis, urinary tract infections, sepsis, influenza, dengue, hepatitis are known to be attributed to the ability of vitamin D to increase antimicrobial peptides (catelicidine and beta-defensins) equipped with antiviral and immunomodulatory activity.
A study conducted by Kim35 showed reduced values of 25 (OH) D (14 ± 8 ng/ml) in patients with community-acquired acute pneumonia. In patients with inflammatory bowel disease, it has been shown that, in the presence of 25 (OH) D levels <20 ng/ml, the administration of vitamin D3 (500 U/day) reduces the incidence of upper respiratory tract infections by two-thirds.
A concentration of 25 (OH) D higher than 38 ng/ml is associated with halving the risk of acute respiratory infections of the respiratory system.36
Grant et al.37 have reported a possible role of vitamin D in the prevention and treatment of coronavirus. According to the authors, vitamin D reduces the risk of respiratory infections through three mechanisms:
The use of nonsteroidal anti-inflammatory drugs (NSAIDs) in the course of COVID-19 infection presents controversial assessments.40 The first studies that tested the efficacy of ibuprofen contraindicate its use due to its association with a higher risk of electrolyte water alteration and aggravated pneumonia. However, there are conflicting opinions regarding the previous concept, as the action of reduction in platelet aggregation could play a protective role on the possible microthrombotic evolution. The use of NSAIDs is still controversial and under discussion in the scientific community.32
The role of corticosteroids in the treatment of viral pneumonia or acute respiratory distress syndrome (ARDS) associated with COVID-19 remains controversial and constantly changing. Corticosteroids act at the transcription level of NF-K beta with a reduction of all cytokine cascades.
It must be remembered that these drugs switch their mechanism of action according to their therapeutic dosage threshold. An immunosuppressive action is obtained for prednisone starting from 25 mg per day up to higher dosages. However, steroids are known to reduce the immune response during viral infections.
Therefore, the opportunity to undergo corticosteroid treatment, the stage of the disease at which to start treatment and the associated immunomodulatory drugs, should be carefully considered. The drug to be used, the dose, the time, and the method of administration are not defined and are still being tested. The current recommendation is to use a mild dose; the dosage should be low to moderate (≤0.5–1 mg/kg per day of methylprednisolone or equivalent); and the duration should be short (≤7 days).32,41
In the more advanced phase of COVID-19, hyperinflammation along with possible serious local and systemic consequences is triggered. This condition represents a negative prognostic factor. At the level of the lungs, manifestations of arterial and venous vasculopathy, thrombosis of the small vessels and evolution toward serious, and sometimes permanent lung lesions (pulmonary fibrosis) are observed.42,43 The final stages of this very serious clinical aspect lead to severe ARDS and in some cases to DIC. During this phase, an alteration was observed in PCR, ferritin, and pro-inflammatory cytokines. Also, increased coagulation parameters are observed, such as levels of fragments of degradation of fibrin D-dimer, consumption of coagulation factors, and thrombocytopenia.44,45
The clinical manifestations and the laboratory findings are similar to that of hemophagocytic lymphohystiocytosis (a rare clinical disease often triggered by a viral infection). The rationale behind an anticoagulant action therefore arose.46
At present, with the knowledge we have acquired on SARS-CoV-2, we can distinguish three clinical phases of the disease with distinct clinical manifestations, pathogenesis, and treatment (Table 2). The data common to all three phases are those on the personal immune response of each patient suffering from SARS-CoV-2. In fact, individuals with a balanced Th1/Th2 concentration probably manifest the pathology in a mild, if not asymptomatic, form. Those who are carrying an imbalance in Th1/Th2 concentration, especially Th17/T Reg, will produce a true “cytokine storm,” which will feed itself and amplify through excessive autophagy at the cellular level.10,11 This amplification process will lead to endothelial damage through which DIC can lead to death.12
Kissler et al.47 hypothesized that the SARS-CoV-2 disease will persist until an effective and tolerated vaccine is found; this will take between 1 and 2 years.48 Hence, in this scenario, therapies through medication are currently in progress and those validated for the treatment of the disease are of enormous importance. The different treatment for each phase requires an early diagnosis and an improvement in home management of patients. These factors could play an important role in treating future cases.49
Our literature review shows that antimalarial drugs should be placed at the very early stages of the disease (phase 1),25,50–53 in order to prevent the evolution toward the cytokine cascade: it also shows that monoclonal antibodies should be used in the second and third phases of the disease characterized by a rapid and exponential growth of IL and cytokine storm that leads to the dramatic events of interstitial pneumonia, multiple organ failure, DIC, and finally death.27,54 Since many therapies performed today are off-label, our opinion that it is important to create shared protocols to establish the optimal timing and duration of these different treatments. The limitation of our study is that it is a narrative review. A systematic review and meta-analysis are also required.
Two multicentre investigations, the Seneca study published in the Lancet in 1995 and the More study, highlighted that Italy has a high incidence of hypovitaminosis D.16,17,36 The prevalence of severe hypovitaminosis among healthy elderly people in Italy, according to the Seneca study,16 is estimated to be 92%, and the highest incidence was seen among individuals hospitalized in institutions and homes for the elderly. These findings might explain why Italy had high mortality rates due to SARS-CoV-2 in hospital institutions compared to other countries. This reflection should lead to a more careful selection of the high-risk population.
The guidelines of the international rheumatology societies are to continue, without interruption, immunomodulatory drug therapies in rheumatic patients who have already used them. At present, this does not seem to have increased the incidence of SARS-CoV-2 in these patients. Indeed, the available studies seem to report a lower percentage of this infectious disease in rheumatic patients.17
Since it is likely that the pandemic infection will continue, there is the obligation to implement all measures in order to prevent infection. In this sense, all measures must be increased, from social distancing to correct use of personal protective equipment and early diagnosis. In addition, pending the development of the desirable vaccine, the challenge will be to identify patients at high risk, both to change their lifestyle and to correct the state of their health through non-pharmacological measures aimed at achieving a correct immuno-balance.
COVID-19 is a very complex systemic disease that can be divided into three different stages with different pathogenesis, treatment, and prognosis. It requires a global multidisciplinary approach, and it is essential to use the medical treatment at the correct pathogenic moment; timing for drug intervention is essential both to control the evolution of the disease and to reduce the possible side effects. Our literature review shows the important role and the therapeutic potential of antirheumatic agents; however, there is a lack of clinical evidence to support their employment and further randomized controlled studies are required. The current challenge is to deliver the available drugs in an optimal combination and at the correct time in the development of this disease.
The authors have declared that no conflicts of interest exist.
There was no funding source available to conduct this study.
We gratefully thank Guido Maria Valentini for the assistance during in the conduction of this study.