Original Article
Nallan CSK Chaitanya1, Danam Reshmapriyanka1, Kandi Pallavi1, Shaik Ameer1, Amurtha Appala2, Avanthi chowdhary3, Tirupathi prabhath1, Marikanti Pota Ratna1, Bodakunta Sai Sowmya1, Chintireddy Vaishnavi1 and Parinita Bontala1
1Department of Oral Medicine and radiology, Panineeya institute of dental sciences, Hyderabad
2Department of Oral Pathology and Microbiology, PMVIDS, Hyderabad
3Department of Conservative dentistry and endodontics
Background: Oral lichen planus (OLP) is a T cell-mediated chronic autoimmune disorder directed against antigens secreted by the basal cell layer, with an incidence of 0.02–0.22% in Indian population and showing female predilection. Stress is considered one of the etiological factors in the causation, progression, and recurrence of this disease.
Objectives: To evaluate the levels of serum cortisol, anxiety, and depression in patients with symptomatic OLP and to correlate the levels of serum cortisol with anxiety and depression.
Methods: Sixty subjects were categorized into two groups. Group A: 30 adults with no history of OLP and no psychological history of anxiety and depression. Group B: 30 patients with clinically and histopathologically diagnosed symptomatic OLP. The subjects in both groups were evaluated for anxiety and depression levels using the Hospital Anxiety and Depression Scale (HADS) questionnaire and serum cortisol levels (8–9 am sample) using the chemiluminiscence method.
Results: Higher depression and anxiety levels were significantly associated with group B with significant P values (P < 0.0001 and <0.0002 respectively) when compared with group A; higher mean serum cortisol levels were seen in group B compared with group A, with P < 0.0001. In group A, a positive correlation was found between depression, anxiety, and serum cortisol levels with non-significant P-value. In group B, a positive correlation was found between depression, anxiety, and serum cortisol levels with a significant P value (P < 0.0001).
Conclusion: Increased levels of depression and anxiety with increased serum cortisol levels were observed in subjects with OLP.
Keywords: oral lichen planus, serum cortisol, depression, anxiety
Key message: The present study demonstrated that the serum cortisol levels were raised in most of the patients having anxiety or depression with OLP, implicating psychosomatic aspect of this chronic disease.
Lichen planus (LP) is a chronic autoimmune disease mediated by T lymphocytes involving the stratified squamous epithelial tissue.1,2 Oral lesions are seen in around 50–70% of patients with LP.3,4 It is commonly seen in Asian population and the age of onset is 3rd and 6th decade of life with a prevalence of 1–2% in general population and 2.6% in Indian population5 with an incidence of 0.02–0.22%,6 and is mostly found in females.7 Clinically, oral lichen planus (OLP) may contain both red and white elements with different textures, which serve as the basis of clinical classification into reticular, papular, plaque-like, bullous, erythematous and ulcerative types.8 Although the etiology remains unknown except for T-cell-mediated chronic inflammation, a myriad of antigen-specific and nonspecific hypotheses have been postulated.9 It could be exacerbated or precipitated due to psychosocial stresses through neuroendocrine and neuroimmunologic mechanisms.10
A direct relationship between OLP and psychological factors have been postulated in the past, suggesting the role of these psychological disturbances, such as anxiety and depression, to be one of the causative factors in the onset and recurrence of OLP.
Many metabolic and endocrine changes occur in conditions involving pain, anxiety, stress, fright, and acute tissue damage. One of them being increase in the levels of serum cortisol. Cortisol, a stress hormone, is a 21-carbon glucocorticoid secreted by the adrenal cortex. It has multiple biological effects and is capable to affect human stress response.11
Many scales aid in knowing the psychological status of patients. In 1983, the Hospital Anxiety and Depression Scale (HADS) was specifically developed for use in physically ill patients.12 It is considered as an acceptable, reliable, valid, and easy-to-use practical tool for psychiatric evaluation and assistance.13 It mainly focuses on anxiety and depression, considered as the two relevant clinical aspects of an emotional disorder, and is less focused on somatic symptoms.14
Literature has focused on the available evidence of stress as one of the cofactors for causation of LP. Therapeutic interventions were carried out based on the results of various studies. In contrast, there are few studies with negative correlation between stress and OLP, which may altogether differ with the concept of therapeutic interventions for stress.
With such contrasting evidence, the present study was aimed to evaluate the levels of serum cortisol along with assessment of anxiety and depression using the HADS questionnaire in patients suffering from OLP, matched with suitable controls.
The institutional ethical review board approved the study protocol. The clinical trial registry identifier was NCT03011658.
A total of 60 subjects were selected from outpatients attending the Department of Oral Medicine and Radiology, Panineeya Mahavidyala Institute of Dental Sciences and Research Centre, Hyderabad, India, and were categorized into two groups: Group A and Group B.
Group A, a control group, comprised 30 patients, not under any medication for any known disease and who visited the Department of Oral Medicine and Radiology for routine dental checkup.
Group B, a case group, included 30 patients with clinically and histopathologically diagnosed symptomatic OLP.
Patients who were willing to participate in the study and with clinical diagnosis and histopathological confirmation of OLP in the age group of 18–90 years of either gender were included in the study. Following patients were excluded from the study: with severe systemic illness; having lesions resembling LP such as contact allergy and lichenoid reaction; patients on corticosteroids and oral contraceptives; and those having endocrine disorders.
Subjects in both the groups were evaluated for anxiety and depression levels using the HADS questionnaire and were asked to reply that was closest to how they were feeling in the past week, and were instructed not to take too much time with an assurance that “immediate is best.”
The HADS questionnaire was translated into regional languages and validated by statistician for non-English speaking patients in order to attain uniformity among participants. The summed-up scores for anxiety and depression separately were obtained with the range between 0 and 7 considered as normal, 8 and 10 as borderline, and 11 and above as being abnormal or having frank or established anxiety/depression.
The subjects were further evaluated for serum cortisol levels. Fasting blood samples were collected between 8 and 9 am under aseptic conditions. Normal range of cortisol is 4.30–22.40 μg/dL and any values above or below this range were considered abnormal. The samples were assessed for serum cortisol levels using chemiluminescence method. ADVIA Centuar XP immunoassay diagnostic system was used for the above purpose. The ADVIA Centaur Cortisol assay is a competitive immunoassay using direct chemiluminescent technology. Cortisol in the patient sample competes with acridinium ester-labeled cortisol in the Lite Reagent for binding to polyclonal rabbit anti-cortisol antibody in the solid phase. The polyclonal rabbit anti-cortisol antibody is bound to monoclonal mouse anti-rabbit antibody, which is covalently coupled to paramagnetic particles in the solid phase.
An inverse relationship exists between the amount of cortisol present in the patient’s sample and the amount of relative light units (RLUs) detected by the system.
Data were summarized as mean ± SD for continuous data, median ± interquartile range (IQR) for score data, and percentage values for categorical data. Comparison between two groups for continuous data was done by unpaired t-test and that for score data was done by Mann–Whitney U test. The relation between two variables for continuous data was done by Karl Pearson’s correlation test, and for score data this was done by Spearman’s rank correlation test; P < 0.05 was considered as statistically significant. The data were analyzed by statistical software IBM SPSS 20.0 version (SPSS Inc., Chicago, IL, USA).
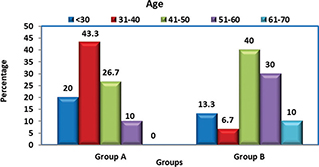
Group A: In this group, the age of the subjects ranged from 18 to 53 years, with a mean age of 37.83 years. Majority of them (43.3%) were in the age range of 31–40 years, followed by 26.7% in the range of 41–50 years. Group B: In this group, the age of subjects ranged from 21 to 67 years, with a mean age of 46.3 years. Majority of them (40%) were in the age range of 41–50 years, followed by 30% in the range of 51–60 years.
Groups A and B: In both groups, there were 22 females and 8 males, making it to 73.3% females and 26.7% males in each group.
Group A: In this group, the range of HADS score was from 2 to 10, with a median of 7. The majority of subjects (83.3%) were normal and 16.7% had borderline depression. There were no subject with established depression.
| Groups | N | Range | Median | IQR | P-value |
|---|---|---|---|---|---|
| Group A | 30 | 2–10 | 7 | 7–3.75 | <0.0001 |
| Group B | 30 | 4–14 | 8 | 12–7 | |
| IQR, interquartile range. | |||||
Group B: In this group, the range of HADS score was from 4 to 14, with a median of 8. Fourty percent of the subjects have shown borderline depression, with 30 % showing established depression and the remaining 30 % were normal. Higher depression levels were significantly associated with group B, with P < 0.0001 when compared with group A.
Group A: In this group, the range of HADS score was from 2 to 10, with a median of 7.50; 50% of the subjects showed borderline anxiety and the other 50% were normal with none showing established anxiety.
| Groups | N | Range | Median | IQR | P-value |
|---|---|---|---|---|---|
| Group A | 30 | 2–10 | 7.50 | 9–6.75 | 0.0002 |
| Group B | 30 | 6–17 | 9.50 | 13.5–8 | |
| IQR, interquartile range. | |||||
Group B: In this group, the range of HADS score was from 6 to 17, with a median of 9.5. The majority (43.3%) of subjects showed established anxiety, followed by 40% with borderline anxiety levels and 16.7% were normal.
Higher anxiety levels were significantly associated with group B, with P < 0.0002 when compared with group A.
Group A: Total 30 patients showed normal serum cortisol levels ranging from 4.41 to 17.9 μg/dL and the mean serum cortisol level was 8.84 μg/dL.
Group B: Out of total 30 patients, 4 patients showed increased serum cortisol levels ranging from 6.37 to 35.74 μg/dL, but the mean serum cortisol was relatively higher with 16.71 μg/dL. Statistical analysis inferred that there was significant difference between group A and group B regarding serum cortisol levels, with P < 0.0001.
Group A: In this group, there were no subjects with abnormal depression. The mean serum cortisol level in 16.7% subjects with borderline depression was 11.44 μg/dL and in the remaining 83.3% subjects, the mean serum cortisol level was found to be 2.27 μg/dL, with nonsignificant P value (0.22).
Group B: In this group, 40% of subjects with borderline depression had a mean serum cortisol level of 14.22 μg/dL, 30% of subjects with established depression had a mean serum cortisol level of 24.72 μg/dL, and the remaining 30% subjects had a mean serum cortisol level of 12.01 μg/dL, with P = 0.001. The mean serum cortisol levels increased with increase in depression levels in both groups.
Group A: In this group, none of the subjects had established anxiety. In all, 50% of the subjects with borderline anxiety had a mean serum cortisol level of 10.16 μg/dL and the remaining 50% subjects had a mean serum cortisol level of 7.53 μg/dL, with significant P = 0.012.
Group B: In this group, 43.3% of subjects with established anxiety had a mean serum cortisol level of 22.65 μg/dL, 40% subjects with borderline anxiety had a mean serum cortisol level of 13.02 μg/dL, and the remaining 16.7% subjects had a mean serum cortisol level of 10.13 μg/dL, with significant P = 0.001.
The mean serum cortisol levels increased with anxiety levels in both groups.
Correlation of Serum Cortisol Levels with Depression and Anxiety in Two Groups (Tables 5 and 6)
Group A: In this group, a positive correlation was found between levels of depression and anxiety and serum cortisol levels, with nonsignificant P value.
Group B: In this group, a positive correlation was found between levels of depression and anxiety and serum cortisol levels, with significant P = 0.0001 and 0.0002.
In the present study, maximum patients were females, with a mean age of 46.3 years. Higher depression and anxiety levels were significantly associated with patients in group B when compared with patients in group A. The mean serum cortisol levels were elevated in group B patients. A positive correlation of serum cortisol levels with depression and anxiety was found in both groups, with statistically significant P-value in group B.
In the present study, the age range of patients with OLP was 21–67 years, with a mean age of 46.3 years, which was in close accordance with the study done by Ingafou et al.15 The mean age was higher in few studies than that in the present study.16,17 In contrast, the mean age was particularly lower in certain studies described in literature than that in the present study.6,18
Out of 30 patients with OLP, maximum were females (73.3%) compared to males (26.7%), which was in accordance with most of the studies.16–18 On contrary, in the studies conducted by Shetty et al.,1 Munde et al.5, and Pati et al.,6 males outnumbered females.
In the present study, higher depression levels were significantly associated in group B (P < 0.0001) when compared with group A, with 40% patients showing borderline depression, followed by 30% with established depression. Higher depression scores were also seen in many of the previous studies.1,13,18–21
The mean depression score in OLP patients was higher as compared with the negative control (healthy patients with no mucosal disease) but almost equal to the positive control (patients with burning mouth syndrome [BMS], atypical facial pain, and myofacial pain dysfunction syndrome).14
However, in the study conducted by Pati,6 73.33% had no evidence of stress and 26.26% had mild depression, which was not in accordance with the present study. This was also supported by a study demonstrating that 88% had no depression, 6% had borderline, and 6% had morbid depression and there was no statistically significant difference in depression scores between OLP patients.22
As demonstrated in this study, higher anxiety levels were significantly associated with group B, with P < 0.0002 when compared with group A. In group B, 43.3% had established anxiety followed by 40% with borderline anxiety and the remaining 16.7% were normal. The median anxiety score was 9.5, which was comparatively much higher than that of group A.
Several other studies have shown higher anxiety levels in patients suffering from OLP, which was in accordance with the present study.1,18–20,22,23,24 In the study conducted by Bansal et al., around 63.2% of patients suffered from borderline or morbid anxiety.17 In a similar way, the mean anxiety score in OLP patients was higher than that in negative controls, but was almost equal to the positive control group.14
Soto Araya et al.20 determined the existing relation between OLP, Recurent Aphthous Stomatitis (RAS), and BMS with psychological alterations. It was observed that stress level was high in patients with RAS and OLP, and hence levels of anxiety were raised in three groups.
There were many contrasting evidences in literature findings with the results of the present study where no statistically significant difference was found when the level of anxiety was compared between OLP and control group.25
Alves et al.27 evaluated emotional characteristics of patients with OLP and demonstrated the presence of anxiety and depression in these patients and a negative impact of disorder on patient’s quality of life as indicated by impairment of physical aspect, vitality, mental health, and social aspect domains. This clearly outlined that psychological treatment may be a vital aspect in the follow-up of these patients.26,27 The role of stress was evaluated before the onset/extension of LP, and that stressful situations, especially related to family, may have a role in the onset and extension of LP lesions.16
In the present study, all 30 subjects in group A had normal serum cortisol levels ranging from 4.41 to 17.9 μg/dL with a mean serum cortisol level of 8.84 μg/dL. In group B, out of total 30 patients, only 4 patients showed increased serum cortisol levels ranging from 6.37 to 35.74 μg/dL but the mean serum cortisol level was relatively high with 16.71 μg/dL. There was significant difference between group A and group B in serum cortisol levels, with P < 0.0001. Although significant difference existed in serum cortisol levels, mean values in both groups were well within the reference range. However, the results couldn’t be attributed to the entire population, as the sample size was relatively small.
The serum cortisol levels in erosive and nonerosive variants of OLP demonstrated that the combined mean serum cortisol value of erosive and nonerosive OLP was found to be higher than that of the control group.1,26 The results of the present study were in accordance with those in literature.
Interplay between stress, serum cortisol level, and OLP was considered as one of the causes of OLP. The present study demonstrated that not all OLP patients had increased serum cortisol levels. Not much comparison was possible pertaining to serum cortisol levels as there were limited number of studies on this particular aspect, clearly implicating the need for further studies regarding the estimation of serum cortisol levels in LP patients. When correlation of serum cortisol levels was done with depression and anxiety, there was a positive correlation in both the groups. Nevertheless, the P-value was not statistically significant in group A, but was statistically significant in group B. Similarly, the results of various studies in literature have suggested a positive correlation between serum cortisol levels and fluctuations in psychological stress as well as between anxiety, depression, and serum cortisol in OLP patients.1,20
The present study demonstrated the role of anxiety and depression being one of the associated factors in causation and prognosis of OLP. Routine counseling sessions with or without pharmacological intervention for psychiatric disorders alongside the treatment of oral conditions in these patients would immensely benefit the prognosis of chronic debilitative mucosal condition.
One of the limitations of the present study is its small sample size, due to which the results might not be attributed to a larger population. Serum cortisol has diurnal variation, with maximum concentrations observed from 7–9 am, and then concentrations coming down during evenings. Hence, to avoid this variation, only morning samples taken between 8 and 9 am were considered. However, the average of both morning and evening serum cortisol levels would reflect accurate levels. Subjects included in the control group who had visited for dental consultation could have caused bias.
The authors have no conflict of interest to declare.
The authors declare that there is no financial support for this study.
Meta Data available on request with the corresponding author.
The student had been carried in compliance with ethical standards.