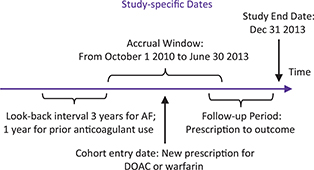
FIG 1. Time Frame Definitions. AF = atrial fibrillation; DOAC = direct-acting oral anticoagulants.
Original Article
Anne Holbrook, MD, PharmD1,2, Richard Morrow, MA3, Agnes Y. Y. Lee, MD, MSc4, Gary Foster, PhD2 and Eleanor Pullenyegum, PhD5
1Division of Clinical Pharmacology & Toxicology, McMaster University, Hamilton, ON, Canada
2Department of Health Research Methods, Evidence and Impact (formerly Clinical Epidemiology and Biostatistics), McMaster University, Hamilton, ON, Canada
3Department of Anesthesiology, Pharmacology and Therapeutics, University of British Columbia, Vancouver, BC, Canada
4Department of Medicine, University of British Columbia, Vancouver, BC, Canada
5Child Health Evaluative Sciences, Hospital for Sick Children, Toronto, ON, Canada
Oral anticoagulants (OACs) are high-priority medications, frequently used with clinically important benefit and serious harm. Our objective was to compare the safety and effectiveness of direct-acting oral anticoagulants (DOACs) versus warfarin in a population where anticoagulation management and DOACs were readily available. A retrospective cohort study of all adults living in British Columbia with a diagnosis of atrial fibrillation and a first prescription for an OAC was conducted. Co-primary outcomes were ischemic stroke and systemic embolism, and major bleeding. Secondary outcomes included a net clinical outcome composite and analysis of discontinuation, switching, and key subgroups. We estimated the effects of treatment using time-to-event models with high-dimensional propensity score adjustment to control confounding. After adjustment for prescribing bias, a cohort (n = 20,113, 43.8% female, mean age 72.4 years) with a mean follow-up of 18.1 months showed that patients taking warfarin tended to be poorer, sicker, and less likely to have a cardiologist prescriber. Outcome event rates were not significantly different for DOACs compared to warfarin [adjusted rate ratio of 1.15 (0.91, 1.46) for systemic embolism, 0.94 (0.82, 1.08) for major bleeding, and 0.98 (0.90, 1.06) for net clinical outcome]. Only the effect of age on net clinical outcome met our strict criteria for predicting which group might be superior. Switch of drug class was associated with increased risk of events (p < 0.003). In this population, we found no difference in important clinical outcomes between warfarin and DOACs. Switching compared to not switching was associated with harm.
Keywords: DOACs, warfarin, atrial fibrillation, cohort study, propensity score adjustment
The comparative effectiveness of oral anticoagulants (OACs) remains a priority research topic because of their widespread use, particularly in elderly populations, their major benefit in preventing morbid and fatal thrombotic events, and their potential for major harm, which is largely bleeding.1,2 More than 7 million prescriptions are dispensed annually for OACs in Canada, with estimates of more than 44 million prescriptions annually in the United States.3,4 The large drug budget impact of direct-acting oral anticoagulants (DOACs), which is estimated to add more than $300 million annually in Canada alone, has kept their comparative effectiveness and safety versus warfarin a top priority for drug policy officials as well.5 Large rigorous randomized trials have been critical to allowing DOACs market access, but cannot address whether their rapid uptake in clinical practice, use in countries where International normalized ratio (INR) monitoring is of relatively high quality and use in all key patient subgroups, generates similar benefits and harms compared to warfarin.6–9 Given that the absolute (as opposed to relative) differences between DOACs and warfarin are quite small, there are several reasons why the advantages of DOACs might not be realized in usual clinical practice.10 These revolve around older patients with multiple comorbidities, adherence issues, confusion over multiple dosage regimens, lack of ready access to antidotes, and lack of substantive advantage for patients on warfarin where INR time in therapeutic range is good.11–17
Population-based health databases with large sample sizes and reliable collection of relevant clinical outcomes may be useful for comparative effectiveness research despite nonrandom allocation, a bias now reduced with innovation in methods of case selection, follow-up, analysis, and adjustment.6,18–22 Previous observational studies comparing DOACs and warfarin have lacked a population-level data coverage (producing potentially biased results) or comprehensive look at outcomes.23–47
Our objective for this study was to clarify the overall comparative effectiveness and safety of DOACs versus warfarin in clinical practice in a population with ready access to INR monitoring and to DOACs. Secondary objectives were to compare and contrast important subgroups and their outcomes and examine the impact of switching drug family.
The study is reported following STROBE (Strengthening the reporting of observational studies in epidemiology) guidelines for cohort studies.48
We used a retrospective cohort design in which the treatment effects of new use of DOACs compared to warfarin were estimated, adjusted by propensity scores, for residents with a diagnosis of atrial fibrillation (AF).
The source population included all BC residents aged 18 years or older (population approximately 3.5 million people). De-identified data extracts from PharmaNet, Medical Services Plan billings (physician payments), the Canadian Institute for Health Information hospital Discharge Abstract Database (DAD), British Columbia Vital Statistics death records, and selected LifeLabs laboratory data results were accessed via Population Data British Columbia secure research servers.49–53 Our sampling frame was British Columbia residents enrolled with the Medical Services Plan during the 12 months before starting an OAC drug (index prescription). Eligibility for inclusion in the cohort required a diagnosis of AF in hospital or medical services data within the 36 months prior to the index prescription.
OAC exposure was determined from dispensed prescription database records, from October 1, 2010 to June 30, 2013 (time frame detailed in Figure 1), to identify new users of warfarin, dabigatran, rivaroxaban, or apixaban. New users were defined as no anticoagulant use during a look-back observation period of 12 months prior to the index prescription date for the OAC. The cohort study flow diagram is shown in Figure 2. The index date was defined as date of the new prescription for OAC. Analyses compared new users of DOAC therapy, as a class and as individual agents, to new users of warfarin (the reference group). Determination of exposure was blinded to patient outcome.

FIG 1. Time Frame Definitions. AF = atrial fibrillation; DOAC = direct-acting oral anticoagulants.
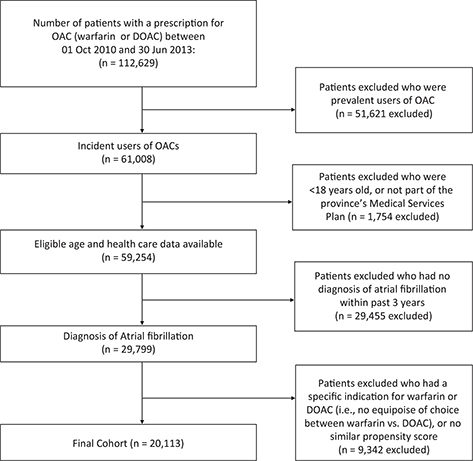
FIG 2. Cohort Study Flow Chart.
The co-primary outcomes, chosen for their clinical importance and their similarity to those in the pivotal trials, were the composite of ischemic stroke and systemic embolism (benefit), and major bleeding was defined as bleeding requiring hospitalization (harm).
Secondary outcomes included the following:
All the clinical outcomes have been validated.55–58
Data codes are available in Appendix 1 (available at: https://rsjh.ca/holbrook/CES-AC_Protocol_Appendices_Jun29_16.pdf).
Outcomes were counted in a follow-up window to the earliest of 24 months post-index prescription, death, exit from British Columbia, the end of the study window, or the occurrence of the relevant study outcome. However, we did not censor follow-up in the analysis of one clinical outcome if a different clinical outcome occurred first (end of follow-up was outcome-specific), and we did not censor at discontinuation of OAC therapy.
We estimated high-dimensional propensity scores using both predefined covariates and covariates empirically selected by an algorithm designed to decrease confounding—details in Appendix 2.59,60
Following the estimation of high-dimensional propensity scores, we excluded patients from the DOAC and warfarin exposure groups who had propensity scores not present in the other exposure group, to exclude patients who would not be comparable to any members of the opposite exposure group. Finally, we restricted the cohort to exclude patients where one OAC group would be contraindicated or overwhelmingly preferred for a patient (based on prior medical history), since this violates the principle of equal chance of exposure to either OAC group. Such medical history included mechanical heart valve, severe chronic kidney disease, and hip or knee replacement.61
The primary analysis was intention to treat, meaning that if the index prescription was for warfarin, then any outcome events in follow-up were attributed to warfarin whether switching occurred or not. This mimicked the conservative, recommended analysis in clinical trials.62 The effects of treatment with DOACs versus warfarin were estimated using generalized linear models with a log link function, assuming a Poisson distribution of the outcome variable. We estimated both crude models and models adjusted by age group, sex, and high-dimensional propensity score decile to control for confounding. We used these models to estimate rates and risk ratios for primary and secondary outcomes, comparing patients exposed to any DOAC to patients exposed to warfarin. In addition, we estimated rates and rate ratios for these outcomes comparing individual DOACs to warfarin. Comparative time to discontinuation was calculated as a ratio of the mean time to discontinuation for DOACs compared to warfarin. This was estimated using an accelerated failure time model, assuming a Weibull distribution for time to failure.
Subgroup analyses were undertaken to raise hypotheses about groups that might do better on warfarin compared to DOACs or vice versa. These included age, sex, rural versus urban location, specialist versus primary care prescriber, history of stroke, renal failure, or congestive heart failure, and comorbidity, clinical prediction score for risk of stroke in patients with AF (CHADs-Vasc) and clinical prediction score for risk of bleeding in patients on OAC (HAS-BLED) scores (definitions in Table 1, details in Appendix 2).
Switching between warfarin and any DOAC was examined as a binary outcome as was each of the three domains of clinical outcomes. If at any point during the study the patient was switched to the other OAC group, they were designated as a switcher. Association between switching status and outcomes does not take timing into account. All analyses were carried out using SAS V9.4.
Ethics approvals were obtained from Hamilton Integrated Research Ethics Board Application #16-643-C and UBC Clinical Research Ethics Board Application #H13-00868 and data sharing agreements with PopData-BC and LifeLabs Medical Laboratory Services. Secure access and storage of data and data linkage were governed by Population Data BC.49–53 All inferences, opinions, and conclusions drawn in this manuscript are those of the authors and do not reflect the opinions or policies of the Data Stewards.
A total of 29,662 patients were enrolled into the study between October 1, 2010 and June 30, 2013. Of the patients, 43.8% were females; the mean age was 72.4 years (standard deviation [SD] 11.7 years); the median family income quintile ranged from $60,000 to $85,000 annually; and 86.6% were residing in urban areas. Selected baseline characteristics of the restricted cohort (n = 20,113) are shown in Table 1, with more detailed baseline characteristics in Appendix 3. To demonstrate the effectiveness of adjustment by sex, age group, and high-dimensional propensity scores decile, we produced a cohort in which we matched new DOAC users with new warfarin users (Appendix 4).
The mean follow-up was 18.1 months (SD 6.74). Over the cohort entry interval, 57.6% of patients initiated OAC therapy with warfarin, 30.1% with dabigatran, 11.9% with rivaroxaban, and 0.4% with apixaban, reflecting the time of market launch and the provincial formulary availability at the time. Rates of comorbidity noted within 3 years of cohort entry were high (details in Table 1). Mean (SD) CHADS-Vasc and HAS-Bled scores were 3.41 (1.70) and 2.11 (1.13), respectively. In terms of interacting drugs, anti-platelets were co-prescribed for 3,056 (15.2%), nonsteroidal anti-inflammatory drugs for 1,701 (8.5%), and antimicrobials for 4,056 (20.2%).
Expressed as unadjusted risk ratios (95% confidence interval [CI]), patients starting a DOAC instead of warfarin were less likely to be with lower income (0.81 [0.78, 0.84]) or have been hospitalized previously for AF (0.70 [0.67, 0.73]), but were more likely to live in an urban location (1.06 [1.05, 1.07]) and to have a low comorbidity score (1.21 [1.18, 1.24]), or have been seen immediately beforehand by a cardiologist (used as surrogate for initial prescriber) (2.41 [2.24, 2.59]).
Table 2 details the primary and secondary outcome results. Co-primary outcome rates of ischemic stroke or systemic embolism, and major bleeding were not significantly different between groups, adjusted rate ratio (aRR) of 1.15 (0.91, 1.46) and 0.94 (0.82, 1.08), respectively. Likewise, the net clinical outcome composite rate (ischemic stroke, systemic embolism, myocardial infarction, pulmonary embolism, major bleeds, or death) was similar between groups (aRR 0.98 [0.90, 1.06]). Net clinical outcome individual component results affirmed that death is an important competing risk for the primary outcomes, as it occurs more frequently than the thromboembolic and bleeding events combined.
| Outcome events | Events | Adjusted rates† (per 100 py) | Crude rate ratio (95% CI) | Adjusted rate ratio (95% CI) | ||||
|---|---|---|---|---|---|---|---|---|
| Warfarin | DOAC | Warfarin | DOAC | Warfarin | DOAC | Warfarin | DOAC | |
| n = 11,578 | n = 8,535 | |||||||
| Primary outcomes | ||||||||
| Ischemic stroke or systemic embolism | 252 | 143 | 1.0 (0.6,1.5) | 1.1 (0.7, 1.7) | 1.00 | 0.78 (0.63,0.95) | 1.00 | 1.15 (0.91,1.46) |
| Major bleeding | 727 | 407 | 3.3 (2.6,4.3) | 3.1 (2.5, 4.0) | 1.00 | 0.77 (0.68,0.87) | 1.00 | 0.94 (0.82,1.08) |
| Fatal bleeding | 53 | 22 | - | - | - | - | - | - |
| Life-threatening bleeding | 134 | 52 | - | - | - | - | - | - |
| Secondary outcomes | ||||||||
| Net clinical benefit (or harm)* | 2,167 | 1,053 | 7.4 (6.3,8.6) | 7.2 (6.2,8.4) | 1.00 | 0.67 (0.62,0.72) | 1.00 | 0.98 (0.90,1.06) |
| Ischemic stroke | 247 | 143 | 0.9 (0.6,1.4) | 1.1 (0.7,1.7) | 1.00 | 0.80 (0.65,0.99) | 1.00 | 1.18 (0.93,1.49) |
| Systemic embolism | 27 | 11 | 0.08 (0.02,0.34) | 0.07 (0.02,0.30) | 1.00 | 0.57 (0.28,1.14) | 1.00 | 0.84 (0.38,1.85) |
| Myocardial infarction | 197 | 124 | 0.5 (0.3,0.9) | 0.6 (0.4,1.1) | 1.00 | 0.87 (0.70,1.09) | 1.00 | 1.21 (0.93,1.56) |
| Pulmonary embolism | 37 | 20 | 0.2 (0.1,0.6) | 0.2 (0.1,0.5) | 1.00 | 0.75 (0.44,1.29) | 1.00 | 0.84 (0.45,1.55) |
| Death from any cause | 1,248 | 528 | 2.9 (2.3,3.6) | 2.8 (2.2,3.6) | 1.00 | 0.59 (0.53,0.65) | 1.00 | 0.99 (0.88,1.11) |
| py = person-years; CI = confidence interval; DOAC = direct-acting oral anticoagulants.
Note: the 89 patients initiating apixaban were excluded due to small numbers. *. Includes ischemic stroke, systemic embolism, MI, pulmonary embolism, major bleeding and death from any cause. †. Adjusted models included adjustment by sex, age group and propensity score decile. | ||||||||
Time to discontinuation was longer for DOACs as a group than for warfarin with a ratio of mean time to discontinuation of 1.52 (1.46, 1.59), with similar results for dabigatran and rivaroxaban as individual agents (Table 3).
Switching OAC family (from warfarin to DOAC or vice versa) was associated with adverse outcomes, with aRRs for switchers of 2.24 (1.46, 3.45), p < 0.0005, for stroke and systemic embolism; 1.41 (1.04, 1.91), p < 0.003, for major bleeding; and 1.54 (1.29, 1.85), p < 0.0001, for net clinical harm.
Forest plots (Figure 3) of the association of key subgroups (age, sex, home location, prescriber, risk factors, etc.) with clinical outcomes did not reveal a subgroup effect for the composite of stroke or systemic embolism. For major bleeding, the interaction p-values suggest that there is a difference in the risk of bleeding between DOAC and warfarin users depending on age group and CHADS-Vasc score category. Age was the only subgroup variable with a significant interaction p-value for net clinical outcome.
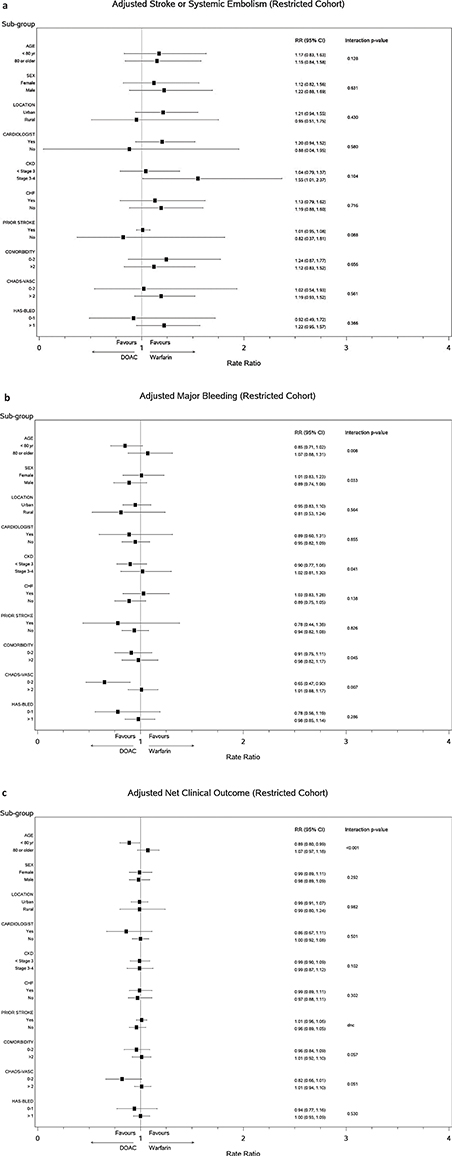
FIG 3. Subgroup Analysis for Clinical Outcomes. (a) Adjusted stroke or systemic embolism (Restricted cohort), (b) Adjusted major bleeding (Restricted cohort), (c) Adjusted net clinical outcome (Restricted cohort).
In our population-based cohort study of the new users of OAC for AF, warfarin was prescribed more frequently than DOACs for older, sicker individuals. We found that rates of primary outcomes (thrombotic events, major bleeding events, and net clinical outcome) were similar between DOACs together or dabigatran and rivaroxaban individually, and warfarin. The composite of net clinical outcome, which includes major OAC-related adverse events and death, is an important summary outcome of combined benefit and harm and shows that death is an important competing risk often ignored in other studies. Although the mean time to discontinuation was longer for DOACs than for warfarin, this result is likely confounded by formulary rules that mandate starting on warfarin with later switch to a DOAC allowed if certain criteria are met. Our subgroup analysis which rigorously adjusted for multiple subgroup testing found that only age was a significant predictor of comparative OAC effect on events, suggesting that adults 80 years or older were likely to suffer fewer net clinical outcome events on warfarin compared to DOACS and vice versa for those younger than 80 years.
One of the novel findings of this study was the highly statistically significant association of switching OAC family compared to not switching, with adverse outcomes. However, future studies should clarify whether adverse outcomes prompt switching or switching OAC family leads to increased adverse outcomes. The latter would support a long-held belief of clinicians that the peri-switching period of OACs is a high-risk period due to variability in anticoagulation effect, adherence with instructions, etc., but may well be confounded by the reasons behind switching as well.
Adjustment for prescribing bias and patient differences between groups rendered our results similar to those found in the individual Phase III randomized trials for each of the DOACs.7–9,63 In recent years as trials accumulate, it appears that DOACs as a group are superior to warfarin for stroke or systemic embolism and major bleeding, particularly intracranial bleeding.64 Apixaban and rivaroxaban but not dabigatran are likely superior to warfarin for myocardial infarction.64 Other observational studies of DOACs individually or as a group versus warfarin have examined only one outcome (i.e., are not comprehensive) or suffer from biases related to a lack of population-level data coverage, major limits on access to DOACs, suboptimal INR management for warfarin or failed to account for death as a competing risk of events.22–47
Our study has several limitations. First, despite efforts to adjust for differences between the groups in predictive factors, this is a retrospective observational study; therefore, bias is always possible due to unmeasured confounders. Second, studies using health administrative data, which themselves are based on real clinical practice, are subject to missing data and coding errors. The main components of this study, important clinical outcomes requiring hospitalization, vital status, medication dispensing, etc., are well validated and known to be complete. However, some of our more specific hypotheses such as INR time in therapeutic range, influence of anemia, or poor renal function could not be assessed given the very high rates of missing laboratory data. A preliminary analysis of community laboratory data from British Columbia suggests that the quality of INR management may be suboptimal but failed to restrict the analysis to maintenance periods.65 Third, delays in data access meant that we were unable to complete an analysis of current prescribing, where apixaban is more prevalent and could be included in comparisons.
In conclusion, after reducing bias and confounding, we found no difference in important clinical outcomes between warfarin and DOACs. Switching compared to not switching between OAC groups in either direction, however, was significantly associated with adverse clinical outcomes. Future research should compare individual DOACs head to head as data accumulate and explore the additional risks associated with switching anticoagulants.
The authors declare no conflict of interest
This work was supported by a grant from the Canadian Institutes for Health Research—Grant # 126150.
After the appropriate ethical approvals and research agreements, the required data were transferred from the Ministry of Health and Lifelabs to PopulationData BC, where they were linked for individual-specific longitudinal analysis, then accessed and analyzed using secure research server access.
Ethics approvals were obtained from Hamilton Integrated Research Ethics Board Application #16-643-C and UBC Clinical Research Ethics Board Application #H13-00868, and data sharing agreements with PopData-BC and LifeLabs Medical Laboratory Services.
(1) Description of High-dimensional Propensity Score Matching
We estimated high-dimensional propensity scores using both predefined covariates and covariates empirically selected by an algorithm which prioritizes covariates based on the potential for controlling confounding according to an assessment of multiplicative bias.1–6 Predefined covariates included indicators for year of cohort entry, neighborhood income quintile, rural residence, residence in long-term care, history of palliative care, visit to cardiologist or internist within 7 days prior to cohort entry, or at least one hospitalization in year prior to cohort entry. We also included indicators for the number of medications used in the year prior to cohort entry, indicators for specific medication use [antacids, antimicrobials, antivirals, nonsteroidal anti-inflammatory drugs, anticoagulants other than direct acting oral anticoagulants (DOACs) or warfarin, and selective serotonin reuptake inhibitors, and antiplatelet agents], alcohol abuse, angina, coronary artery bypass graft, congestive heart failure, dementia, diabetes, hemoglobin ≤100 g/L, hospitalization for atrial fibrillation, hypertension, liver disease, non-hemorrhagic stroke, peripheral artery disease, hospitalization due to major bleeding, stage 3 or 4 chronic kidney disease, previous coronary stents, and transient ischemic attack. Medical covariates were measured in the 3 years prior to cohort entry, hemoglobin level was based on the most recent test in the 180 days prior to cohort entry, and medication use was based on the 120 days prior to cohort entry, unless otherwise indicated.
In addition to predefined covariates, we used the HDPS algorithm to empirically select covariates for the estimation of propensity scores, from the following dimensions (data sources): hospital diagnoses, hospital procedures, physician visit diagnoses, physician visit services (fee items), and medication records. We specified for the algorithm to identify the 200 codes that were most prevalent within each data sources among the study cohort members. The algorithm creates covariates based on the recurrence of these codes. We further specified for the algorithm to retain the top 500 covariates which were estimated to have the highest potential for confounding, based on an assessment of multiplicative bias; both predefined covariates and these empirically selected covariates were used to estimate high-dimensional propensity scores used for the adjustment of analyses.
In the estimation of high-dimensional propensity scores, binary variables were included to indicate when data were missing for predefined covariates.
(2) Subgroup Analysis Methods
Subgroup analyses were undertaken to raise hypotheses about groups that might do better on warfarin compared to DOACs or vice versa. These included age, sex, rural versus urban location, specialist versus primary care prescriber, history of stroke, renal failure, congestive heart failure, comorbidity, CHADs-Vasc score, and HAS-BLED score. Interaction between OAC group (warfarin vs. DOAC) and the subgroup variable when both main effect terms and the interaction term were in the model was assessed for significance. The subgroup estimates and 95% CI were derived from the subgroup term in a model without the interaction term. Because there were 10 subgroups explored, we identified a corrected interaction p-value of 0.005 as the threshold for statistical significance.
| Characteristic | All OACS | Warfarin | All DOACs | Dabigatran | Rivaroxaban | Apixaban | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | |
| Patients | 20,113 | 100 | 11,578 | 57.6 | 8,535 | 42.4 | 6,049 | 30.1 | 2,397 | 11.9 | 89 | 0.4 |
| Age (years), mean (SD) | 72.8 (11.5) | 73.4 (11.3) | 72.1(11.7) | 72.2 (11.9) | 71.9 (11.2) | 72.5 (10.9) | ||||||
| Gender (female) | 8,792 | 43.7 | 5,186 | 44.8 | 3,606 | 42.2 | 2,504 | 41.4 | 1,066 | 44.5 | 36 | 40.4 |
| Family annual income quintile | ||||||||||||
| 1 (<$40,000) | 3,871 | 19.2 | 2,481 | 21.4 | 1,390 | 16.3 | 964 | 15.9 | 419 | 17.5 | 7 | 7.9 |
| 2 ($40,000–$60,000) | 3,906 | 19.4 | 2,373 | 20.5 | 1,533 | 18.0 | 1,102 | 18.2 | 417 | 17.4 | 14 | 15.7 |
| 3 ($60,000–$85,000) | 3,900 | 19.4 | 2,297 | 19.8 | 1,603 | 18.8 | 1,120 | 18.5 | 467 | 19.5 | 16 | 18.0 |
| 4 ($85,000–$125,000) | 3,999 | 19.9 | 2,142 | 18.5 | 1,857 | 21.8 | 1,303 | 21.5 | 530 | 22.1 | 24 | 27.0 |
| 5 (>$125,000) | 4,191 | 20.8 | 2,111 | 18.2 | 2,080 | 24.4 | 1,512 | 25.0 | 540 | 22.5 | 28 | 31.5 |
| Missing | 246 | 1.2 | 174 | 1.5 | 72 | 0.8 | 48 | 0.8 | 24 | 1.0 | 0 | 0.0 |
| Place of residence | ||||||||||||
| Home–Urban | 17,438 | 86.7 | 9,779 | 84.5 | 7,659 | 89.7 | 5,412 | 89.5 | 2,165 | 90.3 | 82 | 92.1 |
| Home–Rural | 2,675 | 13.3 | 1,799 | 15.5 | 876 | 10.3 | 637 | 10.5 | 232 | 9.7 | 7 | 7.9 |
| Long-term care | 470 | 2.3 | 406 | 3.5 | 64 | 0.7 | 43 | 0.7 | 21 | 0.9 | 0 | 0.0 |
| Palliative care | 89 | 0.4 | 69 | 0.6 | 20 | 0.23 | 15 | 0.2 | 5 | 0.2 | 0 | 0.0 |
| Missing | 498 | 2.5 | 263 | 2.3 | 235 | 2.8 | 182 | 3.0 | 51 | 2.1 | 2 | 2.2 |
| Prescriber physician specialty (visit within 7 days pre-cohort entry) | ||||||||||||
| Cardiology | 2,670 | 13.3 | 962 | 8.3 | 1,708 | 20.0 | 1,158 | 19.1 | 520 | 21.7 | 30 | 33.7 |
| Internal medicine | 2,374 | 11.8 | 1,239 | 10.7 | 1,135 | 13.3 | 793 | 13.1 | 329 | 13.7 | 13 | 14.6 |
| Missing | 1,102 | 5.5 | 336 | 2.9 | 766 | 9.0 | 490 | 8.1 | 269 | 11.2 | 7 | 7.9 |
| Patient’s medical history (within 3 years pre-cohort entry) | ||||||||||||
| Hypertension | 14,827 | 73.7 | 8,584 | 74.1 | 6,243 | 73.1 | 4,414 | 73.0 | 1,759 | 73.4 | 70 | 78.7 |
| CKD, stage 3–4 | 4,418 | 22.0 | 2,771 | 23.9 | 1,647 | 19.3 | 1,157 | 19.1 | 466 | 19.4 | 24 | 27.0 |
| Liver disease | 691 | 3.4 | 418 | 3.6 | 273 | 3.2 | 191 | 3.2 | 78 | 3.3 | 4 | 4.5 |
| Non-hemorrhage stroke | 2,686 | 13.4 | 1,754 | 15.1 | 932 | 10.9 | 715 | 11.8 | 209 | 8.7 | 8 | 9.0 |
| Transient ischemic attack | 1,596 | 7.9 | 952 | 8.2 | 644 | 7.5 | 481 | 8.0 | 159 | 6.6 | 4 | 4.5 |
| Alcohol abuse | 448 | 2.2 | 307 | 2.7 | 141 | 1.7 | 102 | 1.7 | 37 | 1.5 | 2 | 2.2 |
| Dementia | 879 | 4.4 | 593 | 5.1 | 286 | 3.4 | 208 | 3.4 | 77 | 3.2 | 1 | 1.1 |
| Congestive heart failure | 5,900 | 29.3 | 3,873 | 33.5 | 2,027 | 23.7 | 1,461 | 24.2 | 544 | 22.7 | 22 | 24.7 |
| Diabetes | 6,039 | 30.0 | 3,701 | 32.0 | 2,338 | 27.4 | 1,661 | 27.5 | 661 | 27.6 | 16 | 18.0 |
| Peripheral artery disease | 433 | 2.2 | 284 | 2.5 | 149 | 1.7 | 95 | 1.6 | 52 | 2.2 | 2 | 2.2 |
| Myocardial infarction | 617 | 3.1 | 369 | 3.2 | 248 | 2.9 | 176 | 2.9 | 70 | 2.9 | 2 | 2.2 |
| Angina | 3,971 | 19.7 | 2,306 | 19.9 | 1,665 | 19.5 | 1,176 | 19.4 | 468 | 19.5 | 21 | 23.6 |
| Previous coronary stents | 411 | 2.0 | 240 | 2.1 | 171 | 2.0 | 109 | 1.8 | 60 | 2.5 | 2 | 2.2 |
| Coronary artery bypass graft | 281 | 1.4 | 185 | 1.6 | 96 | 1.1 | 70 | 1.2 | 26 | 1.1 | 0 | 0.0 |
| AF hospitalization/ED | 7,188 | 35.7 | 4,748 | 41.0 | 2,440 | 28.6 | 1,823 | 30.1 | 601 | 25.1 | 16 | 18.0 |
| Romano comorbidity score (diagnoses within 3 years prior) | ||||||||||||
| 0 to 2 | 11,574 | 57.5 | 6,115 | 52.8 | 5,459 | 64.0 | 3,822 | 63.2 | 1,578 | 65.8 | 59 | 66.3 |
| 3 to 5 | 6,383 | 31.7 | 3,972 | 34.3 | 2,411 | 28.2 | 1,743 | 28.8 | 643 | 26.8 | 25 | 28.1 |
| ≥6 | 2,156 | 10.7 | 1,491 | 12.9 | 665 | 7.8 | 484 | 8.0 | 176 | 7.3 | 5 | 5.6 |
| Major bleed hospitalization | 983 | 4.9 | 615 | 5.3 | 368 | 4.3 | 272 | 4.5 | 95 | 4.0 | 1 | 1.1 |
| Lab results (most recent in 1 to 180 days before cohort entry) | ||||||||||||
| INR, missing | 18,754 | 93.2 | 10,777 | 93.1 | 7,977 | 93.5 | 5,641 | 93.3 | 2,255 | 94.1 | 81 | 91.0 |
| INR, available | 1,359 | 6.8 | 801 | 6.9 | 558 | 6.5 | 408 | 6.7 | 142 | 5.9 | 8 | 9.0 |
| Hemoglobin, low | 160 | 2.0 | 106 | 2.6 | 54 | 1.4 | 42 | 1.6 | 12 | 1.1 | 0 | 0.0 |
| Hemoglobin, missing | 12,208 | 60.7 | 7,509 | 64.9 | 4,699 | 55.1 | 3,397 | 56.2 | 1,257 | 52.4 | 45 | 50.6 |
| Hemoglobin, available | 7,905 | 39.3 | 4,069 | 35.1 | 3,836 | 44.96 | 2,652 | 43.8 | 1,140 | 47.6 | 44 | 49.4 |
| CHA2DS2-VASc score | ||||||||||||
| 0 to 2 | 6,056 | 30.1 | 3,113 | 26.9 | 2,943 | 34.5 | 2,067 | 34.2 | 846 | 35.3 | 30 | 33.7 |
| 3 to 5 | 11,986 | 59.6 | 7,082 | 61.2 | 4,904 | 57.5 | 3,474 | 57.4 | 1,376 | 57.4 | 54 | 60.7 |
| ≥6 | 2,071 | 10.3 | 1,383 | 11.9 | 688 | 8.1 | 508 | 8.4 | 175 | 7.3 | 5 | 5.6 |
| HAS-BLED score | ||||||||||||
| 0 to 1 | 5,689 | 28.3 | 2,966 | 25.6 | 2,723 | 31.9 | 1,926 | 31.8 | 769 | 32.1 | 28 | 31.5 |
| 2 to 3 | 12,265 | 61.0 | 7,191 | 62.1 | 5,074 | 59.4 | 3,592 | 59.4 | 1,428 | 59.6 | 54 | 60.7 |
| ≥4 | 2,159 | 10.7 | 1,421 | 12.3 | 738 | 8.6 | 531 | 8.8 | 200 | 8.3 | 7 | 7.9 |
| Mean (SD) # prescription drugs(within 1 year pre-cohort entry) | 9.1 (5.7) | 9.6 (5.9) | 8.3 (5.2) | 8.3 (5.2) | 8.4 (5.1) | 8.0 (4.6) | ||||||
| Concurrent interacting medications (prescribed within 12 days of index OAC prescription) | ||||||||||||
| Anti-platelet drugs (ASA, clopidogrel, prasugrel, ticagrelor, or ticlopidine) | ||||||||||||
| Single antiplatelet drug | 2,807 | 14.0 | 1,760 | 15.2 | 1,047 | 12.3 | 768 | 12.7 | 271 | 11.3 | 8 | 9.0 |
| 2 or more antiplatelet drugs | 249 | 1.2 | 148 | 1.3 | 101 | 1.2 | 67 | 1.1 | 34 | 1.4 | 0 | 0.0 |
| NSAIDS (excluding ASA) | 1,701 | 8.5 | 972 | 8.4 | 729 | 8.5 | 509 | 8.4 | 214 | 8.9 | 6 | 6.7 |
| Any other anticoagulant | 306 | 1.5 | 279 | 2.4 | 27 | 0.3 | 14 | 0.2 | 13 | 0.5 | 0 | 0.0 |
| Antimicrobials | 4,056 | 20.2 | 2,478 | 21.4 | 1,578 | 18.5 | 1,115 | 18.4 | 449 | 18.7 | 14 | 15.7 |
| Antacid medications | 4,539 | 22.6 | 2,763 | 23.9 | 1,776 | 20.8 | 1,247 | 20.6 | 505 | 21.1 | 24 | 27.0 |
| SSRIs | 1,548 | 7.7 | 995 | 8.6 | 553 | 6.5 | 394 | 6.5 | 153 | 6.4 | 6 | 6.7 |
| Selected antivirals | 835 | 4.2 | 525 | 4.5 | 310 | 3.6 | 229 | 3.8 | 78 | 3.3 | 3 | 3.4 |
| AF = atrial fibrillation; OAC = oral anticoagulants; DOAC = direct-acting oral anticoagulants; SD = standard deviation; CKD = chronic kidney disease; ED = emergency department; INR = international normalized ratio; NSAID = nonsteroidal anti-inflammatory drugs; ASA = acetylsalicylic acid; SSRI = selective serotonin reuptake inhibitors. | ||||||||||||
| Characteristic | Unmatched cohort | Matched cohort* | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Warfarin | All DOACs | Warfarin | All DOACs | |||||||||
| n | % | n | % | n | % | n | % | |||||
| Patients | 11,578 | 8,535 | 8,501 | 8,501 | ||||||||
| Age at initiation in years, mean (SD) | 73.4 (11.3) | 72.1 (11.7) | 72.1 (11.5) | 72.2 (11.6) | ||||||||
| Gender (female) | 5,186 | 44.8 | 3,606 | 42.2 | 3,587 | 42.2 | 3,587 | 42.2 | ||||
| Income quintile | ||||||||||||
| 1 | 2,481 | 21.4 | 1,390 | 16.3 | 1,473 | 17.3 | 1,385 | 16.3 | ||||
| 2 | 2,373 | 20.5 | 1,533 | 18.0 | 1,580 | 18.6 | 1,528 | 18.0 | ||||
| 3 | 2,297 | 19.8 | 1,603 | 18.8 | 1,534 | 18.0 | 1,602 | 18.8 | ||||
| 4 | 2,142 | 18.5 | 1,857 | 21.8 | 1,802 | 21.2 | 1,846 | 21.7 | ||||
| 5 | 2,111 | 18.2 | 2,080 | 24.4 | 2,041 | 24.0 | 2,069 | 24.3 | ||||
| Missing | 174 | 1.5 | 72 | 0.8 | 71 | 0.8 | 71 | 0.8 | ||||
| Place of residence | ||||||||||||
| Urban | 9,779 | 84.5 | 7,659 | 89.7 | 7,601 | 89.4 | 7,625 | 89.7 | ||||
| Rural | 1,799 | 15.5 | 876 | 10.3 | 900 | 10.6 | 876 | 10.3 | ||||
| Residence in long-term or palliative care | ||||||||||||
| Long-term care | 406 | 3.5 | 64 | 0.7 | 66 | 0.8 | 64 | 0.8 | ||||
| Palliative care | 69 | 0.6 | 20 | 0.23 | 21 | 0.2 | 20 | 0.2 | ||||
| Missing | 263 | 2.3 | 235 | 2.8 | 117 | 1.4 | 233 | 2.7 | ||||
| Specialty of physician visited in 0 to 7 days before cohort entry | ||||||||||||
| Cardiology | 962 | 8.3 | 1,708 | 20.0 | 1,679 | 19.8 | 1,679 | 19.8 | ||||
| Internal medicine | 1,239 | 10.7 | 1,135 | 13.3 | 1,165 | 13.7 | 1,133 | 13.3 | ||||
| Missing | 336 | 2.9 | 766 | 9.0 | 232 | 2.7 | 764 | 9.0 | ||||
| History of disease in 0 to 1,095 days before cohort entry | ||||||||||||
| Hypertension | 8,584 | 74.1 | 6,243 | 73.1 | 6,210 | 73.1 | 6,224 | 73.2 | ||||
| Chronic kidney disease, stage 3–4 | 2,771 | 23.9 | 1,647 | 19.3 | 1,627 | 19.1 | 1,644 | 19.3 | ||||
| Liver disease | 418 | 3.6 | 273 | 3.2 | 285 | 3.4 | 271 | 3.2 | ||||
| Non-hemorrhage stroke | 1,754 | 15.1 | 932 | 10.9 | 967 | 11.4 | 929 | 10.9 | ||||
| Transient ischemic attack | 952 | 8.2 | 644 | 7.5 | 708 | 8.3 | 644 | 7.6 | ||||
| Alcohol abuse | 307 | 2.7 | 141 | 1.7 | 158 | 1.9 | 140 | 1.6 | ||||
| Dementia | 593 | 5.1 | 286 | 3.4 | 293 | 3.4 | 285 | 3.4 | ||||
| Congestive heart failure | 3,873 | 33.5 | 2,027 | 23.7 | 2,061 | 24.2 | 2,023 | 23.8 | ||||
| Diabetes | 3,701 | 32.0 | 2,338 | 27.4 | 2,366 | 27.8 | 2,332 | 27.4 | ||||
| Peripheral artery disease | 284 | 2.5 | 149 | 1.7 | 133 | 1.6 | 149 | 1.8 | ||||
| Myocardial infarction | 369 | 3.2 | 248 | 2.9 | 227 | 2.7 | 247 | 2.9 | ||||
| Angina | 2,306 | 19.9 | 1,665 | 19.5 | 1,734 | 20.4 | 1,660 | 19.5 | ||||
| Previous coronary stents | 240 | 2.1 | 171 | 2.0 | 153 | 1.8 | 170 | 2.0 | ||||
| Coronary artery bypass graft | 185 | 1.6 | 96 | 1.1 | 99 | 1.2 | 96 | 1.1 | ||||
| Hospitalization or ED visit for atrial fibrillation in the past 3 years | 4,748 | 41.0 | 2,440 | 28.6 | 2,514 | 29.6 | 2,434 | 28.6 | ||||
| Romano comorbidity score in 0 to 1,095 days before cohort entry | ||||||||||||
| 0 to 2 | 6,115 | 52.8 | 5,459 | 64.0 | 5,251 | 61.8 | 5,429 | 63.9 | ||||
| 3 to 5 | 3,972 | 34.3 | 2,411 | 28.2 | 2,540 | 29.9 | 2,408 | 28.3 | ||||
| ≥6 | 1,491 | 12.9 | 665 | 7.8 | 710 | 8.4 | 664 | 7.8 | ||||
| Hospitalization due to major bleed in 0 to 1,095 days before cohort entry | 615 | 5.3 | 368 | 4.3 | 358 | 4.2 | 365 | 4.3 | ||||
| Lab test results, most recent in 1 to 180 days before cohort entry | ||||||||||||
| INR, missing (not available) | 10,777 | 93.1 | 7,977 | 93.5 | 8,065 | 94.9 | 7,944 | 93.4 | ||||
| Hemoglobin, low | 106 | 0.9 | 54 | 0.6 | 52 | 0.6 | 54 | 0.6 | ||||
| Hemoglobin, missing (not available) | 7,509 | 64.9 | 4,699 | 55.1 | 4,690 | 55.2 | 4,684 | 55.1 | ||||
| CHA2DS2-VASc score | ||||||||||||
| 0 to 2 | 3,113 | 26.9 | 2,943 | 34.5 | 2,883 | 33.9 | 2,922 | 34.4 | ||||
| 3 to 5 | 7,082 | 61.2 | 4,904 | 57.5 | 4,921 | 57.9 | 4,892 | 57.5 | ||||
| ≥6 | 1,383 | 11.9 | 688 | 8.1 | 697 | 8.2 | 687 | 8.1 | ||||
| HAS-BLED score | ||||||||||||
| 0 to 1 | 2,966 | 25.6 | 2,723 | 31.9 | 2,567 | 30.2 | 2,705 | 31.8 | ||||
| 2 to 3 | 7,191 | 62.1 | 5,074 | 59.4 | 5,219 | 61.4 | 5,060 | 59.5 | ||||
| ≥4 | 1,421 | 12.3 | 738 | 8.6 | 715 | 8.4 | 736 | 8.7 | ||||
| Interacting medications in 0 to 120 days before cohort entry | ||||||||||||
| Anti-platelet drugs: | ||||||||||||
| Single agent (ASA, clopidogrel, prasugrel, ticagrelor, ticlopidine) | 1,760 | 15.2 | 1,047 | 12.3 | 1,103 | 13.0 | 1,042 | 12.3 | ||||
| ≥2 agents (e.g., 2 single agents or combination ASA-dipyridamol) | 148 | 1.3 | 101 | 1.2 | 106 | 1.2 | 100 | 1.2 | ||||
| NSAIDS (excluding ASA) | 972 | 8.4 | 729 | 8.5 | 647 | 7.6 | 725 | 8.5 | ||||
| Any other anticoagulant | 279 | 2.4 | 27 | 0.3 | 34 | 0.4 | 27 | 0.3 | ||||
| Antimicrobials | 2,478 | 21.4 | 1,578 | 18.5 | 1,581 | 18.6 | 1,573 | 18.5 | ||||
| Antacid medications | 2,763 | 23.9 | 1,776 | 20.8 | 1,798 | 21.2 | 1,771 | 20.8 | ||||
| SSRIs | 995 | 8.6 | 553 | 6.5 | 548 | 6.4 | 551 | 6.5 | ||||
| Selected antivirals | 525 | 4.5 | 310 | 3.6 | 314 | 3.7 | 310 | 3.6 | ||||
| Number of distinct prescription drugs in 0 to 365 days before cohort entry, mean (SD) | 9.6 (5.9) | 8.3 (5.2) | 8.3 (5.1) | 8.3 (5.2) | ||||||||
| DOAC = direct-acting oral anticoagulants; SD = standard deviation; INR = international normalized ratio; ED = emergency department; NSAID = nonsteroidal anti-inflammatory drugs; ASA = acetylsalicylic acid; SSRI = selective serotonin reuptake inhibitors.
*Matched on sex, age group (18–39, 40–49, 50–59, 60–64, 65–69, 70–74, 75–79, 80–84, and ≥85 years), and high-dimensional propensity score within caliper of ±0.05. | ||||||||||||