Original Research
A SHORTENED TREATMENT WITH ROSEMARY TEA (ROSMARINUS OFFICINALIS) INSTEAD OF POWDER DECREASES INSULIN RESISTANCE AND SERUM GLUCOSE IN PATIENTS WITH DIABETES MELLITUS TYPE 2 (T2D)
Sol María Quirarte-Báez1, Ana Lourdes Zamora-Perez2, Claudia Araceli Reyes-Estrada3, Rosalinda Gutiérrez-Hernández4, Martha Sosa-Macías5, Carlos Galaviz-Hernández5, Gloria Guillermina Guerrero Manríquez6 and Blanca Patricia Lazalde-Ramos7*
1Instituto Mexicano del Seguro Social, Zacatecas, Zacatecas, México
2Instituto de Investigación en Odontología, Centro Universitario de Ciencias de la Salud, Universidad de Guadalajara, Jalisco, México
3Maestría en Ciencias de la Salud, Unidad Académica de Medicina Humana, Universidad Autónoma de Zacatecas, Zacatecas, México
4Licenciatura en Nutrición, Unidad Académica de Enfermería, Universidad Autónoma de Zacatecas, Zacatecas, México
5Instituto Politécnico Nacional, Centro Interdisciplinario De Investigación para el Desarrollo Integral Regional, Unidad Durango, Durango, México
6Unidad Académica de Ciencias Biológicas, Universidad Autónoma de Zacatecas, Zacatecas, México
7Maestría en Ciencia y Tecnología Química, Laboratorio de Etnofarmacología, Unidad Académica de Ciencias Químicas, Universidad Autónoma de Zacatecas, Zacatecas, México
*Corresponding author: blancalazalde@gmail.comSubmitted: August 14, 2019. Accepted: October 15, 2019. Published: December 3, 2019.DOI: 10.15586/jptcp.v26i4.634
ABSTRACT
Background
Rosemary leaves powder has been reported to reduce in a dose-dependent manner, glucose levels, lipid profile and lipid peroxidation in humans. However, patients should ingest high doses of powder contained in capsules. This formulation constitutes the intake of 10 capsules per day, so the active metabolite must first, be released and then absorbed (for which, rosemary leaf powder must be mixed with gastric juice).
Aim
Evaluate whether a shortened dose and time of treatment as well as the pharmaceutical presentation in rosemary tea (Rosmarinus officinalis) instead of powder have a therapeutic effect in the treatment of T2D.
Method
The complementary therapy with Rosemary tea (2g/1 litre of water per day) were evaluate on resistance to insulin, oxidative stress, biochemical parameters and anthropometric measurements in forty patients T2D under treatment with metformin and/or glibenclamide afther giving your authorization through informed consent.
Results
The data indicated that Rosemary tea intake after 90 days, statistically decreased (p < 0.05) anthropometric parameters like the body mass index and waist-hip ratio. Remarkably, this treatment decreased the percentages of glycated hemoglobin, insulin resistance, and the pancreatic β-cell function and lastly, a significant difference in lipid peroxide levels was found.
Conclusion
These data show that shortening time and dose, as well as changing the formulation of the Rosemary plant constitutes a promising treatment for drug-resistant T2D patients.
Keywords: diabetes mellitus; hypoglycemia; insulin resistance; oxidative stress; Rosmarinus officinalis
Diabetes mellitus type 2 (T2D) is considered a serious public health problem due to the high increase in incidence and prevalence.1 The main clinical feature of T2D is hyperglycemia, which results from defects in insulin secretion, cellular resistance to the action of insulin, or both, creating an inflammatory alteration of pancreatic β cells or a resistance to the action of insulin in different tissues.2 Indeed, this autoimmune chronic disease is a leading cause of blindness, renal failure, myocardial infarction, stroke, and the amputation of lower limbs.3,4 Current therapy is set for each patient according to their economical budget.5–7 The most accepted therapy is based on pharmacological drugs, which include oral hypoglycemic drugs (e.g., sulfonylureas, meglitinides, biguanides, thiazolidinediones, acarbose, and miglitol). Insulin therapy is used in case of drug therapy failure.8 In other cases, T2D patients select “alternative therapies” such as medicinal plants. However, it has been observed that medicinal plants or their extracts can optimize glucose metabolism and the integral condition of T2D patients, not only by their hypoglycemic effects but also by improving their lipid profile, antioxidant status, and capillary function.9,10 One example of these medicinal plants is Rosmarinus officinalis, known as rosemary, which has been used for diabetes treatment. Although this plant is native from the Mediterranean Basin, it has a worldwide distribution. The properties of this plant reside in the richness of active principles that have an effect on almost all organs of the human body.11,12 Moreover, it is a rich source of phenolic phytochemicals having significant antioxidant, anti-inflammatory, hypoglycemic, hypolipidemic, hypotensive, anti-atherosclerotic, antithrombotic, hepatoprotective, and hypocholesterolemic effects.13 In vivo studies have shown that rosemary reduces glucose levels in rabbits and rats with induced diabetes.14–17 According to the literature, it has been demonstrated that doses of 2, 5, and 10 g/day of rosemary leaves powder reduce in a dose-dependent manner glucose levels, lipid profile, and lipid peroxidation in randomly selected human participants.18 In an independent study, it has been reported that the intake of 3 g/day of powder for 4 weeks reduces in 9% fasting blood glucose and generates favorable changes in lipid profiles.19 However, this formulation constitutes the intake of 10 capsules per day, representing a disadvantage for patients due to the amount that they have to take in order to have a therapeutic effect. Therefore, in the present work, we sought to investigate whether by shortening the dose, time of treatment, as well as changing the pharmaceutical presentation of the Rosemary plant, a therapeutic effect in T2D could be observed.
MATERIAL AND METHODS
Population of Study
We studied 40 patients diagnosed with T2D, of which 67.5% were female and 13 (32.5%) were male. The mean age of the patients was 56.3 ± 9.97 years. The study group included 45% of the patients under pharmacological treatment with metformin, 17.5% with glibenclamide, and 37.5% with metformin and glibenclamide. From the total number of patients, 70% reported having a first-line genetic load for diabetes and 27.5% of the patients were known to have dyslipidemia. In addition, each patient underwent a clinical history, with emphasis on the development of diabetes, time of evolution, detection method, type of diet, and physical activity. All patients received an alternative therapy with rosemary tea in a dose of 2 g/L/day.
The participants gave their informed written consent. The research was carried out in accordance with the Declaration of Helsinki and it was approved by the Research and Ethics Committee of the “Hospital General 450” (registration No. 023), by the Ministry of Health of the state of Durango, and by the state´s Bioethics Committee (registration No. 003/CEB 15), belonging to the Ministry of Health of the state of Zacatecas.
Preparation of Rosemary Tea
Each patient was provided with a box containing 30 sachets of rosemary leaves (2 g each). The rosemary leaves employed were acquired in Plantas Medicinales de América, S.A. de C.V. México, D.F., and then, packed and shipped to the laboratory of ethnopharmacology at the Autonomous University of Zacatecas, according to the Official Mexican Norm NOM-072-SSA1-1993. The patients were instructed to add a bag in a liter of boiling water (boiled for 3 min) and then pass through a sieve to remove the rosemary leaves. The patients were instructed to consume it as drinking water during the day.
Anthropometric Measures
Weight was measured using an electronic digital scale with a capacity of 130 kg. Height was measured using a stadiometer with a length of 2 m, divided into centimeters and subdivided into millimeters.
Waist circumference was measured in the horizontal plane midway in the distance of the superior iliac crest and the lower margin of the last rib, under the clothes, and at the end of a normal exhalation, using a flexible and inelastic measuring tape. The hip was measured at the greatest circumference of the gluteal region.
All measurements were made before initiating phytopharmacological therapy (basal value) and at 90 days of the therapy.
Blood Samples
The blood sample was obtained by venipuncture in the flexor zone of the elbow before the intake of rosemary tea (baseline sample) and at 90 days of phytopharmacological therapy.
Biochemical Testing Measurements
Biochemical profile analyses were performed at the Biomedical Research Diabetes Clinic belonging to the Ministry of Health of Durango. The measurements were made by enzymatic methods. Quantification of insulin was carried out through the enzyme immunosorbent assay for the quantitative detection of antibodies anti-insulin (MexLab®).
Determination of Lipid Peroxides
Malondialdehyde (MDA) concentrations were measured as thiobarbituric acid reactive substances (TBARS) according to a modified version of the procedure described by Yagi.20 In brief, 0.3 mL serum was mixed with 2 mL 1/12 NH2SO4 in a centrifuge tube and gently shaken. Then, 0.3 mL of 10% phosphotungstic acid was added to the tube in addition to 1 mL of thiobarbituric acid (0.6%). The tube was then heated in a boiling water bath for 1 h. The samples were cooled to room temperature. The resulting chromogen was extracted with 1.3 mL n-butyl alcohol by vigorous shaking. The organic phase was separated by centrifugation at 1600 × g for 10 min, and its absorbance was recorded at a wavelength of 530 nm. The level of absorbance was converted intro nmol/mL MDA from a standard curve generated with 1,1,3,3-tetraethoxypropane (Sigma Chemical, St. Louis, MO).
Homeostatic Model Assessment
The insulin resistance score (Homeostasis Model Assessment [HOMA]) was calculated with the formula: fasting serum insulin (μU/mL) and fasting plasma glucose (mmol/L)/22.5, as described by Matthews et al.21
Statistical Analysis
The results are presented as mean ± standard deviation. The comparisons among baseline value (before starting the alternative therapy) and 90 days of therapy with rosemary tea were made with the Wilcoxon and/or paired t-test. Comparisons for categorical variables were performed using chi-squared and/or Fisher’s exact test. A Pearson’s correlation analysis was performed to test the relationship between biochemical indicators and insulin resistance, weight, body mass index (BMI), and waist to hip ratio. All tests were performed using the statistical program SPSS v.20, Chicago, IL; P-values <0.05 were considered statistically significant.
Data Availability Statement
The data types used to support the findings of this study are included within the article and are restricted by the Research and Ethics Committee of the “Hospital General 450” (Secretary of Health of the state of Durango, Mexico) to protect patient privacy. Data are available from the corresponding author, Dr Lazalde Ramos, Campus UAZ Siglo XXI, Edif. L1, 3er piso, Carretera Zacatecas-Guadalajara Km 6, Ejido la Escondida, 98160 Zacatecas, México. Phone: (+52) (492) 1702977, email address; blancalazalde@gmail.com), for researchers who meet the criteria for access to confidential data.
RESULTS
Rosemary Tea Intake Reduced the Anthropometric Measurements of T2D Patients
To gain further insight into the study of the potential of the phytopharmacological treatment of T2D disease based on rosemary tea intake, Table 1 shows the anthropometric measurements (weight, BMI, waist, and/or hip) from the T2D patients’ post-therapy with rosemary tea; weight and BMI did not decrease significantly. In contrast, waist and hip (6.07–5.57%) decreased significantly (P < 0.000) at 90 days post-intake of rosemary tea.
TABLE 1. Anthropometric and Index Homeostasis Model Assessment (HOMA) Values of T2D Patients before and after Intake of Rosemary Tea
| |
Before Therapy with Rosemary Tea (X ± SD) |
At 90 Days Post-Intake of Rosemary Tea (X ± SD) |
|
Percentage Decrease or Increase (%) |
P |
| Weight (kg) |
74.43 ± 14.24 |
73.46 ± 14.71 |
↓ |
1.30 |
NS |
| Body mass index (kg/m2) |
27.54 ± 4.40 |
27.19 ± 4.41 |
↓ |
1.27 |
NS |
| Waist (cm) |
91.38 ± 9.71 |
85.83 ± 9.64 |
↓ |
6.07 |
0.000 |
| Hip (cm) |
103.52 ± 10.72 |
97.75 ± 7.94 |
↓ |
5.57 |
0.000 |
| Waist-to-hip ratio |
0.886 ± 0.058 |
0.879 ± 0.060 |
↓ |
0.79 |
NS |
| Glucose (mg/dL) |
181.18 ± 80.75 |
162.96 ±72.89 |
↓ |
10.05 |
NS |
| HbA1c(%) |
9.09 ± 2.72 |
7.72 ± 1.82 |
↓ |
15.07 |
0.000 |
| Insulin resistance (mUI/mL) |
2.29 ± 0.98 |
1.12 ± 0.75 |
↓ |
51.09 |
0.000 |
| Insulin sensitivity (%) |
48.66 ± 12.47 |
98.48 ± 20.21 |
↑ |
102.38 |
0.000 |
| β-cell function (%) |
54.06 ± 30.45 |
39.50 ± 29.95 |
↓ |
26.93 |
0.003 |
| The comparisons among baseline values (before starting the alternative therapy) and 90 days of therapy with rosemary tea were performed with the Wilcoxon and/or paired t-test depending on the normality of the data. X: mean; SD: standard deviation; NS: not significant. |
Rosemary Tea Ingestion Reduced Glucose and Insulin Resistance
Next, since one of the major concerns in T2D includes glucose levels and insulin resistance, the effect of the ingestion of rosemary tea on these parameters was investigated. From Table 1, it is evident that there is a decrease in the percentage of glycosylated hemoglobin (9.09–7.72%) and in the insulin resistance (2.29–1.12 mU/mL) (P < 0.000) and also in the pancreas with values for β-cell functionality (54.06–39.59%) (P < 0.003). Therefore, there was an increase in the percentage of insulin sensitivity (48.66–98.48%) (P < 0.000) in T2D patients (Table 1).
No Effect Was Observed in the Lipid Profile of T2D Patients after rosemary Tea Ingestion; however, It Did Decrease Lipid Peroxidation
Previous data from the literature have shown that the intake of 3 g of rosemary powder during 4 weeks generates favorable lipid profiles.19 Therefore, cholesterol, triglycerides, high, and low lipoproteins were determined in the blood of the T2D patients. Interestingly, under the rosemary tea formulation, no significant differences were found while comparing the basal value with respect to the values obtained 90 days post-intake of rosemary tea (Table 2). However, rosemary tea intake significantly decreased MDA levels in 30 days from 0.789 ± 0.364 to 0.375 ± 0.306 nM/mL, while maintaining this decrease in relation to time, in 60 days (0.344 ± 0.238) and in 90 days (0.365 ± 0.176 nM/mL) (Figure 1).
TABLE 2. Lipid Profile of Patients with T2D before and after Intake of Rosemary Tea
| |
Before Therapy with Rosemary Tea (X ± SD) |
At 90 Days Post-Intake of Rosemary Tea (X ± SD) |
|
Percentage Decrease or Increase (%) |
P |
| Cholesterol (mg/dL) |
204.27 ± 40.75 |
196.59 ± 34.04 |
↓ |
3.75 |
NS |
| Triglycerides (mg/dL) |
198.75 ± 104.16 |
183.62 ± 81.39 |
↓ |
7.61 |
NS |
| High-density lipoproteins (mg/dL) |
33.03 ± 0.88 |
32.71 ± 0.95 |
↓ |
0.96 |
NS |
| Low-density lipoproteins (mg/dL) |
131.09 ± 24.54 |
126.54 ± 27.61 |
↓ |
3.46 |
NS |
| Very low-density lipoproteins (mg/dL) |
170.78 ± 40.61 |
165.00 ± 35.14 |
↓ |
3.38 |
NS |
| The comparisons among baseline values (before starting the alternative therapy) and 90 days of therapy with rosemary tea were performed with the Wilcoxon and/or paired t-test depending on the normality of the data. X: mean; SD: standard deviation; NS: not significant. |
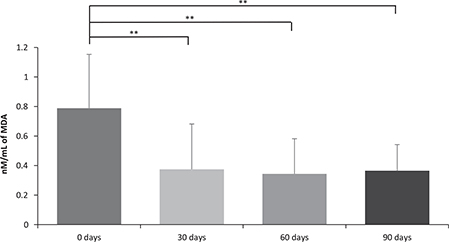
FIG 1. Effect of Rosemary Tea Intake on Lipid Peroxidation. Differences between the different time periods were evaluated with a variance analysis (ANOVA) for repeated measures applying a Bonferroni adjustment for multiple comparisons. The comparisons were: a0 days versus 30 days; b0 days versus 60 days; c0 days versus 90 days; d30 days versus 60 days; e30 days versus 90 days; f60 days versus 90 days. P = 0.0001a,b,c.
In the Table 3 shows the comparison by Fisher’s exact test of the percentage of patients at high risk of diabetic complications before consuming rosemary tea and 90 days post-consumption. The number of patients with triglyceride values >150 mg/dL decreased by 15.62% and the number of patients with Low-Density Lipoprotein Cholestero c-LDL>100 mg/dL decreased by 7.14%.
TABLE 3. Percentage of Diabetic Complications in High-Risk Patients
| |
Before Therapy with Rosemary Tea |
At 90 Days Post-Intake of Rosemary Tea |
|
Percentage Decrease or Increase (%) |
P |
| Glycated hemoglobin (High risk >8.0%) |
54.55% |
21.88% |
↓ |
59.89 |
0.01 |
| Glucose>130 mg/dL |
75.76% |
62.50% |
↓ |
17.50 |
NS |
| Triglycerides>150 mg/dL |
66.67% |
56.25% |
↓ |
15.62 |
NS |
| Cholesterol>200 mg/dL |
54.55% |
56.25% |
↑ |
3.11 |
NS |
| High-density lipoproteins <40 mg/dL |
100% |
100% |
-- |
--- |
NS |
| Low-density lipoproteins> 100 mg/dL |
90.32% |
83.87% |
↓ |
7.14 |
NS |
| Very low-density lipoproteins> 40 mg/dL |
100% |
100% |
-- |
--- |
NS |
| Values are expressed as percentages. Fischer’s exact test was used. NS: not significant. |
Correlation between the Biochemical and Anthropometric Parameters after 90-Day Post-Ingestion of Rosemary Tea
The analysis of the correlation coefficients between the anthropometric (Table 1) and biochemical (Table 3) parameters indicated that insulin resistance, percentage of insulin sensitivity, and percentage of β-cell function showed a high and moderate correlation with serum glucose levels (r = 0.813, −0.591, −0.625, respectively; P = 0.000) and HBA1c (r = 0.639, −0.567, −0.359, respectively; P ≤ 0.001). Insulin resistance also showed a high correlation with cholesterol (r = 0.421; P = 0.000), triglycerides (r = 0.235; P = 0.038), Very Low-Density Lipoprotein Cholesterol (c-VLDL) (r = 0.348; P = 0.002), and moderate correlations with MDA (r = 0.284; P = 0.012), while the percentage of insulin sensitivity correlated only with cholesterol levels (r = −0.260; P = 0.022). The percentage of β-cell function with cholesterol (r = −0.324; P = 0.004), c-VLDL (r = −0.325; P = 0.004), and MDA (r = −0.281; P = 0.013).
The Antidiabetic Drug Glibenclamide Decreased Total Cholesterol and LDL
Figure 2 shows the results from patients who received complementary therapy with pharmacological treatment, showing a statistical difference in the parameters of total cholesterol and LDL.
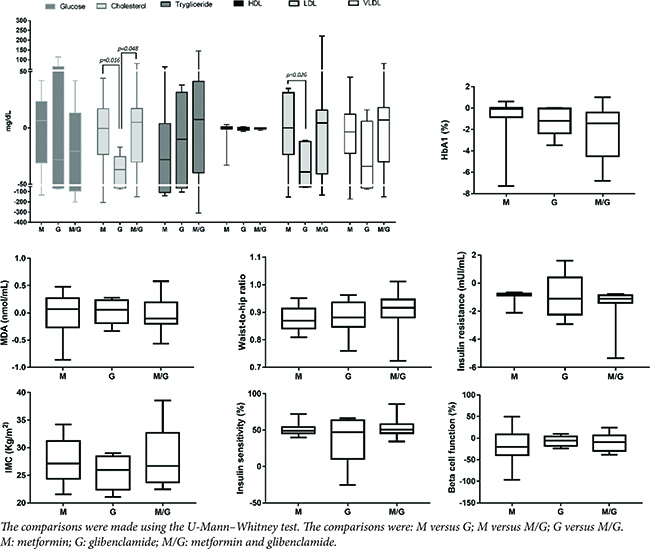
FIG 2. Effect of Pharmacological Treatment on the Parameters Evaluated in Patients under Complementary Therapy with Romero Tea.
Patients with pharmacological therapy with glibenclamide showed a significant decrease in the total cholesterol concentration in relation to the metformin and metformin and glibenclamide groups, likewise the group that received glibenclamide significantly decreased the LDL concentration in relation to the metformin group (Figure 2)
DISCUSSION
In this study, we report that the ingestion of rosemary tea decreases some of the anthropometric measurements (related with the protective effect on fat accumulation and hypertrophy inhibition) and significantly reduces insulin resistance, serum glucose, and lipid peroxidation in T2D patients from the state of Durango in Mexico.
As reported by the literature, it has been shown that rosemary leaves powder at a dose of 3 g/day during 4 weeks exerts a positive effect on the reduction of glucose levels, lipid profile, and lipid peroxidation.18 However, patients should ingest high doses of powder contained in capsules. This formulation constitutes the intake of 10 capsules per day, which should first release the active metabolites and then absorbed (rosemary leaf powder mixed with gastric juice). Herein, we sought to evaluate whether a shortened dose and time of treatment as well as pharmaceutical presentation of rosemary tea instead of powder could have a therapeutic effect on T2D treatment. The results indicated that a shortened dose, time, and changing the pharmaceutical presentation of rosemary tea (2 g/L/day) had a favorable effect on the different biochemical parameters measured in patients with T2D (Tables 1 and 2, Figure 1)
After rosemary tea intake, patients had an HbA1c percentage of 9.09 ± 2.72%, while post rosemary ingestion, it was 7.72 ± 1.82%, thus leading to a decrease in the percentage of patients who were at high risk of diabetic complications (HBA1c >8%) by 59.89% (Table 1). Although the average serum glucose levels did not decrease statistically after the intake of rosemary tea, the percentage of patients with glucose levels >130 mg/dL decreased by 15.62% after rosemary tea therapy (Table 2). It is highly possible that serum glucose is directly related to the diet of the last 24 h, so that patients could present diet transgressions. No statistical difference was found in the lipid profile of patients prior and post phytopharmacological therapy with rosemary. However, a slight decrease in triglyceride (7.61%), cholesterol (3.75%), LDL lipoprotein (3.46%), and VLDL lipoprotein levels (3.38%) was observed after the complementary therapy with rosemary tea (Tables 2 and 3). The observed effects of the rosemary tea intake in T2D patients could be due to the fact that rosemary significantly reduces blood glucose levels16,17 and potentially increases liver glycolysis and fatty acid oxidation through the activation of cyclic adenosine monophosphate (cAMP) and peroxisome proliferator-activated receptor (PPARs) pathways.22–24 The intake of rosemary tea in the patients studied decreased waist and hip circumference, and this decrease could be attributed to the fact that rosemary contains carnosic acid which has been reported to produce a protective effect on fat accumulation and inhibits hypertrophy of adipocytes from white adipose tissue conditioning the decrease of tissue.13,25–28 Moreover, the data reported are consistent with those reported by Labban et al., and the authors evaluated doses of 2, 5, and 10 g of rosemary leaves powder in healthy individuals. They found that the intake of rosemary at doses of 2 g/day did not significantly decrease serum glucose and that the hypoglycemic effect of rosemary was dose-dependent, as the 5 and 10 g doses statistically decreased serum glucose levels, with this decrease being greater in the 10 g dose, without any toxic effect.18 Moreover, Al Jamal et al. reported a 9% decrease in serum glucose in patients with T2D with dyslipidemia after ingesting 3 g of rosemary leaves per day for 4 weeks and 21% in healthy subjects.19 In addition, Yun et al. demonstrated that the phenolic diterpenes from the aqueous extract of rosemary suppress the responsiveness to cAMP of gluconeogenic genes.29 Stefanon et al. reported that rosemary extract modulates the differentiation of human adipocytes and significantly interferes with adipogenesis and lipid metabolism.30
The hypolipidemic potential of rosemary leaves has been demonstrated by lowering blood levels of triglycerides, cholesterol, LDL lipoprotein, and increasing HDL lipoprotein in vivo.31,32
Similarly, rosemary phenolic compounds have been reported to reduce blood cholesterol concentrations in rats with hypercholesterolemia33 and the administration of rosemary extract enriched with carnosic acid improved the lipid profile in Zucker rats.34
Bustanji et al. reported that rosemary extract inhibits lipase-sensitive hormone (HSL), which impinges on the metabolic switch between glucose and free fatty acids (FFAs)35; while rosemary intake significantly decreased lipoperoxidation in the patients studied at 30 days, maintaining this decrease in relation to the time of rosemary tea intake.
The findings described in this article show that insulin resistance and sensitivity as well as pancreatic β-cell function were significantly correlated with serum glucose levels and HbA1c, indicating that there is a decrease in hyperinsulinism.36
Patients with pharmacological therapy with metformin and complementary therapy with rosemary tea significantly decreased total cholesterol and LDL levels compared to the metformin and metformin and glibenclamide group. It has been reported that glibenclamide therapy significantly decreases the levels of serum total cholesterol after 15 and 30 days of therapyand increases HDL serum levels.37 Glibenclamide promoted the HDL-independent cholesterol efflux by decreasing esterified cholesterol and increasing the release of free cholesterol and secretion of apolipoprotein E into the medium.38
In summary, these data show that rosemary soluble active principles in water at a low dose constitute a promising treatment for T2D and resistant T2D patients.
ACKNOWLEDGMENTS
We thank patients from the Diabetes Clinic of the Secretary of Health of the state of Durango for their enthusiastic participation in the realization of this project as well as its Director Dr. Oscar Vladimir Campos Moreno and the Clinical Laboratory Director QFB Cesar Mijares Figueroa for the facilities granted.
FUNDING
This project did not receive any type of financing.
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
REFERENCES
- Federación Internacional de Diabetes. Atlas de la diabetes de la FID (6th edn.). Ginebra: FID; 2001, p. 160.
- Aguilar-Salinas CA, Rojas R, Gómez-Pérez FJ, et al. Prevalence and characteristics of early-onset type 2 diabetes in Mexico. Am J Med 2002;113:569–74.
- World Health Organitazion Ginebra. World diabetes report. WHO: Ginebra; 2016.
- ENSANUT 2012. Encuesta Nacional de Salud y Nutrición 2012. https://ensanut.insp.mx/
- Domínguez-Alonso E, Seuc A, Díaz O, Aldana D. La carga de la diabetes en Cuba, período 1990–2005. Rev Cubana Endocrinol 2008;22:23–8.
- Sikorski C, Luppa M, Kaiser M, et al. The stigma of obesity in the general public and its implications for public health-a systematic review. BMC Public Health 2011;11:664–1.
- Dávila-Torres J, González-Izquierdo JJ, Barrera-Cruz A. Panorama de la obesidad en México. Rev Med Inst Mex Seguro Soc 2015;53:240–9.
- Hunt JV, Dean RT, Wolff SP. Hydroxyl radical production and autoxidative glycosylation glucose autoxidation as the cause of protein damage in the experimental glycation model of diabetes mellitus and aging. Biochem J 2012;256:205–12.
- Yeh GY, Eisenberg DM. Kaptchuk TJ, et al. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 2003;26:1277–94.
- Dodda D, V Ciddi. Plants used in the management of diabetic complications. Indian J Pharm Sci 2014;76:97–106.
- Almela L, Sánchez-Muñoz B, Fernández-López JA, et al. Liquid chromatographic- mass spectrometric analysis of phenolics and free radical scavenging activity of rosemary extract from different raw material. J Chromatogr A 2006;1120(1–2):221–9.
- Tschinggerl C, Bucar F. Investigation of the volatile faction of Rosemary infusion extracts. Sci Pharm 2010;1:483–92.
- Hassani FV, Shirani K, Hosseinzadeh H. Rosemary (Rosmarinus officinalis) as a potential therapeutic plant in metabolic syndrome: A review. Naunyn Schmiedebergs Arch Pharmacol 2016;389:931–49.
- Lipina C, Hundal HS. Carnosic acid stimulates glucose uptake in skeletal muscle cells via a PME-1/PP2A/PKB signalling axis. Cell Signal 2014;26:2343–9.
- Bower AM, Real Hernandez LM, Berhow MA, et al. Bioactive compounds from culinary herbs inhibit a molecular target for type 2 diabetes management, dipeptidyl peptidase IV. J Agric Food Chem 2014;62:6147–58.
- Bakirel T, Bakirel U, Keleş OU, et al. In vivo assessment of antidiabetic and antioxidant activities of Rosemary (Rosmarinus officinalis) in alloxan-diabetic rabbits. J Ethnopharmacol 2008;116:64–73.
- Ramadan KS, Khalil OA, Danial EN, et al. Hypoglycemic and hepatoprotective activity of Rosmarinus officinalis extract in diabetic rats. J Physiol Biochem 2013;69:779–83.
- Labban L, Mustafa US, Ibrahim YM. The effects of Rosemary (Rosmarinus officinalis) leaves powder on glucose level, lipid profile and lipid peroxidation. Inte J Clin Med 2014;5:207–304.
- Al Jamal A. Effect of Rosemary (Rosmarinus officinalis) on lipid profiles and blood glucose in human diabetic patients (type-2). Afr J Biochem Res 2014;8:147–50.
- Yagi K. Simple procedure for specific assay of lipid hydroperoxides in serum or plasma. Methods Mol Biol 1998;108:101–6.
- Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9.
- Herzig S, Long F, Jhala US, et al. CREB regulates hepatic gluconeo-genesis through the coactivator PGC-1. Nature 2001;413:179–83.
- Rau O, Wurglics M, Paulke A, et al. Carnosic acid and carnosol, phenolic diterpene compounds of the labiate herbs Rosemary and sage, are activators of the human peroxisome proliferator-activated receptor gamma. Planta Med 2006;72:881–7.
- Tu Z, Moss-Pierce T, Ford P, et al. Rosemary (Rosmarinus officinalis L.) extract regulates glucose and lipid metabolism by activating AMPK and PPAR pathways in HepG2 cells. J Agric Food Chem 2013;61:2803–10.
- Ninomiya K, Matsuda H, Shimoda H, et al. Carnosic acid, a new class of lipid absorption inhibitor from sage. Bio Org Med Chem Lett 2004;14:1943–6.
- McCue PP, Shetty K. Inhibitory effects of rosmarinic acid extracts on porcine pancreatic amylase in vitro. Asia Pac J Clin Nutr 2004;13:101–6.
- Koga K, Shibata H, Yoshino K, et al. Effects of 50% ethanol extract from Rosemary (Rosmarinus officinalis) on α-glucosidase inhibitory activity and the elevation of plasma glucose level in rats, and its active compound. J Food Sci 2006;71:S507–12.
- Wang T, Takikawa Y, Satoh T, et al. Carnosic acid prevents obesity and hepatic steatosis in ob/ob mice. Hepat Res 2011;41:87–92.
- Yun YS, Noda S, Shigemori G, et al. Phenolic diterpenes from Rosemary suppress cAMP responsiveness of gluconeogenic gene promoters. Phytother Res 2013;27:906–10.
- Stefanon B, Pomari E, Colitti M. Effects of Rosmarinus officinalis extract on human primary omental preadipocytes and adipocytes. Exp Biol Med 2015;240:884–95.
- Naidu KA, Thippeswamy NB. Inhibition of human low density lipoprotein oxidation by active principles from spices. Mol Cell Biochem 2002;229:19–23.
- Devi R, Sharma DK. Hypolipidemic effect of different extracts of Clerodendron colebrookianum Walp in normal and high-fat diet fed rats. J Ethnopharmacol 2004;90:63–8.
- Alfonso MS, de O Silva AM, Carvalho EB, et al. Phenolic compounds from Rosemary (Rosmarinus officinalis L.) attenuate oxidative stress and reduce blood cholesterol concentrations in diet-induced hypercholesterolemic rats. Nutr Metab 2013;10:19–24.
- Romo-Vaquero M, Yáñez-Gascón MJ, García Villalba R, et al. Inhibition of gastric lipase as a mechanism for body weight and plasma lipids reduction in Zucker rats fed a Rosemary extract rich in carnosic acid. PLoS One 2012;7:e39773.
- Bustanji Y, Issa A, Mohammad M, et al. Inhibition of hormone sensitive lipase and pancreatic lipase by Rosmarinus officinalis extract and selected phenolic constituents. J Med Plants Res 2010;4:2235–42.
- León-Pedroza JI, González-Tapia LA, Del Olmo-Gil E, et al. Low-grade systemic inflammation and the development of metabolic diseases: From the molecular evidence to the clinical practice. Cir Cir 2015;83:543–51.
- Singh T, Singh S, Bhullar GS. The effect of sulphonylurea therapy on serum total cholesterol and high density lipoprotein cholesterol. J Indian Med Assoc 1992;90(10):259–61.
- Nobusawa A, Taniguchi T, Fujioka Y, et al. Glibenclamide inhibits accumulation of cholesteryl ester in THP-1 human macrophages. J Cardiovasc Pharmacol 2000;36(1):101–8.

