FIG 1. ROC curve.
Original Research
Mohamed Shawky Elfarargy*1, Mohamed S. El Farargy1, Marwa Mohamed Atef2, Omnia Safwat El-Deeb2, Radwa Mahmoud Elsharaby3, and Hany Abd Elfattah Elhady4
1Department of Pediatrics, College of Medicine, Jouf and Tanta University, KSA and Egypt
2Department of Medical Biochemistry, Faculty of Medicine, Tanta University, Tanta, Egypt
3Department of Clinical Pathology, Faculty of Medicine, Tanta University, Tanta, Egypt
4Department of Surgery, College of Medicine, Jouf and Azhar University, Cairo, Egypt
Background
Necrotizing enterocolitis (NEC) is a frequent serious disease of the digestive system in neonates. It is considered as an important cause of serious neonatal complication and death. Therefore, its early suspicion and proper management are important.
Aim
Early and sensitive detection of neonatal NEC through determination of levels of fecal calprotectin (FCP), serum levels of procalcitonin (PCT), high-sensitivity C-reactive protein (hs-CRP), epithelial neutrophil activating peptide-78 (ENA-78), and interleukin 18 (IL-18).
Method
This prospective case control study was conducted in Tanta University Hospital from June 2016 to March 2018. The study included 20 healthy neonates (control group) and 20 NEC newborn patients. They were all subjected to the measurement of levels of FCP and serum levels of hs-CRP, PCT, ENA-78, IL-18, Malondialdehyde (MDA), and total antioxidant capacity (TAC). Receiver operating characteristic (ROC) curve analysis was conducted for FCP, ENA-78, PCT, hs-CRP, and IL-18.
Results
The study found a detectable increase in FCP level and serum levels of hs-CRP, PCT, ENA-78, IL-18, and MDA in NEC group in comparison to their levels in the control group. Also, it found a detectable decline in the levels of TAC in comparison to its level in the control group.
Conclusion
FCP, ENA-78, and PCT can be considered as early markers for diagnosis of NEC.
Keywords: necrotizing enterocolitis; neonate; fecal calprotectin; biomarkers; procalcitonin; interleukin 18
Necrotizing enterocolitis (NEC) is a severe disease of the digestive system that mainly presents in preterm neonates.1 NEC is one of the most dangerous neonatal diseases causing multiple complications to the neonates and may cause death if discovered late.2 Early symptoms of NEC cannot be easily distinguished from other similar conditions, and thus various studies were conducted for early diagnosis and appropriate management for better prognosis.3 The etiology of NEC may be due to placental insufficiency, chorioamnionitis, and altered bacterial colonization.4
Proinflammatory cytokines have been supposed to be present in the pathogenesis of NEC.5 Interleukin 18 (IL-18) has an important role in inflammation and is present in many inflammatory diseases of the small intestine.6 Epithelial neutrophil activating peptide 78 (ENA-78) also has an important regulatory role in neutrophil influx during inflammation. These mediators are important markers in serum during sepsis.7
It was documented that reactive oxygen species (ROS) have an important function in gastrointestinal damage and affection of the mucosa of the digestive tract in NEC.8 Procalcitonin (PCT), the calcitonin precursor, is an important mediator produced by monocytes. It started to rise very early, within 3 hours following the affection by the bacterial endotoxin as most of the biomarkers need several hours or even days to rise in response to bacterial toxin so 3 h in procalcitonin is considered very early which allowed us to diagnose the case early with early treatment with better prognosis.9–11
In addition, a high number of C-reactive proteins (CRPs) are observed in response to bacterial infection and neonatal sepsis; high-sensitivity C-reactive protein (hs-CRP) has a vital diagnostic value in neonatal infection as newborn infants cannot react to several infections by secreting large quantity of mediators of inflammation, and there is a small rise in CRP in responding to infection which unlike adults who secrete large quantity of mediators of inflammation.12,13
Calprotectin is considered as a protein where the calcium and the zinc bind to it and this protein represents about 60% of protein which is soluble in cytosol in neutrophil granulocytes. It is characterized by its stability even after a week of storage at room temperature because it resists proteolysis, and this facilitates its determination in feces.14 Increased quantity of fecal calprotectin (FCP) correlates with increased turnover of leukocytes in the intestinal barrier and granulocyte migration toward intestinal lumen.15,16 FCP can be used also as a rapid, available, and noninvasive detector of NEC.17 In light of the above considerations, we aimed to predict rapid suspicion of NEC through determination of levels of fecal (FCP), serum levels of PCT, hs-CRP, ENA-78, and IL-18.
This is a prospective case–control study which was conducted among newborns admitted to the neonatal intensive care unit of Tanta University Hospital from June 2016 to February 2018, and the enrolled newborns were classified as control (healthy newborns) group (n=20) and NEC (patients) group (n=20). The inclusion criteria were abdominal distension, blood in stool, and pneumatosis intestinalis on X-ray,3 while the exclusion criteria were congenital infection (TORCH), congenital malformation, hypoxic ischemic encephalopathy, and intracranial hemorrhage.
This study was approved by the Ethics Committee of the Faculty of Medicine, Tanta University (clearance number: 3469/12/16) in 2016. Written informed consent was obtained from the parents of all neonates.
All neonates had been done all of the following: complete history taking and clinical examination. The studied groups had laboratory investigations in the form of complete blood count, CRP, electrolytes (Na, K, and so on), PH, and abdominal radiology.
NEC is assessed according to modified bell staging.18 Stage I (A, B) or suspected NEC includes neonates who present with the minimal clinical picture. There may be anorexia, vomiting, increased digestive tract residuals which is proved by insertion of orogastric tube, multiple degree of distended abdomen, or occult blood in the stool. Stage II (A, B) or proven NEC shows the very characteristic radiological sign of NEC which is pneumatosis intestinalis. There may be some manifestation like cessation of respiration, decrease in the heart rate, and other manifestation of sepsis like poor activity.
Stage III (A, B) or advanced NEC infants show deteriorated vital signs or marked gastrointestinal bleeding. Pneumoperitoneum may be present.
Under complete aseptic conditions, 5 mL of blood from the neonates was collected from both groups and was transferred gently into a dry sterile centrifuge tube, left to clot at room temperature, centrifuged at 1,200 × g for 5 min, and serum was collected, and was frozen at −80°C after collection till analysis.
Serum Malondialdehyde (MDA) level was calculated at 532 nm through an extinction of the coefficient of the present MDA–TBA complex, which is 1.56 × 105 M-1 cm-1, which was done according to Ohkawa et al.19 Serum total antioxidant capacity (TAC) was also estimated using the colorimetric commercial kit supplied by Biodiagnostic, Egypt.
Serum hs-CRP was assessed by ELISA kit, which was offered and available from Biosystem, Barcelona, Spain, as the results of Serum hs-CRP may differ from method to other. Measurement of serum PCT using the RayBio® (RayBiotech Company, USA) of the human PCT was performed. Serum interleukin-18 (IL-18) level measurement through the available RayBio® of the human IL-18 was done, and serum epithelial neutrophil activating peptide-78 (ENA-78) level assessment using the ELISA kit by R&D Systems Inc., USA, was also performed according to the manufacturer’s instructions.
Stool specimen was gathered in sterile plastic well-capped containers and directly stored frozen at −20°C until analysis. Detection of FCP in stool was done using Immundiagnostic AG ELISA kit (Bensheim, Germany) according to the manufacturer’s instructions.
Data are expressed as mean ± SD range. The t test was used for group comparisons of normally distributed variables. The computer program SPSS (SPSS version 21, IBM, Armonk, NY, United States of America) was used for every statistical calculation, and version 21 was used in the statistical analysis. P<0.05 was considered as significant.
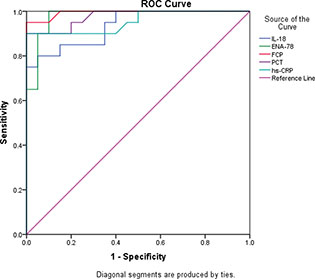
The neonates in this study were formulated into two groups: 20 healthy newborns as control group and 20 neonates with NEC as the patients group. In Table 1, a significant positive correlation was revealed among hs-CRP, PCT, IL-18, ENA-78, FCP, and MDA, while TAC showed significant negative correlation with the studied parameters. In Table 2, the sensitivity and specificity of FCP, ENA-78, PCT, hs-CRP, and IL-18 for detection of NEC were evaluated using receiver operating characteristic (ROC) curve (Figure 1).
| FCP | hs-CRP | PCT | IL-18 | ENA-78 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| FCP | ||||||||||
| hs-CRP | 0.895 | <0.001* | ||||||||
| PCT | 0.953 | <0.001* | 0.803 | <0.001* | ||||||
| IL-18 | 0.928 | <0.001* | 0.954 | <0.001* | 0.853 | <0.001* | ||||
| ENA-78 | 0.923 | <0.001* | 0.947 | <0.001* | 0.856 | <0.001* | 0.980 | <0.001* | ||
| MDA | 0.921 | <0.001* | 0.887 | <0.001* | 0.888 | <0.001* | 0.912 | <0.001* | 0.927 | <0.001* |
| TAC | −0.877 | <0.001* | −0.881 | <0.001* | −0.843 | <0.001* | −0.886 | <0.001* | −0.912 | <0.001* |
FCP, fecal calprotectin; hs-CRP, high sensitivity C-reactive protein; PCT, procalcitonin; IL-18, interleukin 18; ENA-78, epithelial neutrophil activating peptide-78; MDA, Malondialdehyde; TAC, total antioxidant capacity; r, Pearson’s correlation coefficient. *P<0.05 is significant. |
||||||||||
The optimal cutoff point of FCP was 227.55, with sensitivity 95% and specificity 95%, and in ENA-78 the cutoff point was 159.67, with sensitivity 90% and specificity95%. PCT cutoff point was 9.35, with sensitivity 90% and specificity 95%.
As shown in Table 2, the hs-CRP cut-off point was 2.89, with sensitivity 90% and specificity 90%, and in IL-18 the cutoff point was 71.2, with sensitivity 85% and specificity 85%,. From these results we find that FCP, ENA-78, and PCT can be considered as valuable biomarkers in NEC diagnosis being key players involved in NEC pathogenesis.
In Table 3, the demographic data of both studied groups are shown. No significant differences concerning sex, age, and gestational age were found between both studied groups.
In Table 4, The NEC group shows significant higher MDA level and significant lower TAC level when compared with control group.
| Groups | Range | Mean ± SD | t-test | P | |
|---|---|---|---|---|---|
| MDA (nmol/mL) | Patients | 0.73–0.89 | 0.79 ± 0.13 | 2.818 | 0.001* |
| Control | 0.19–0.32 | 0.24 ± 0.04 | |||
| TAC (mmol/L) | Patients | 0.57–0.91 | 0.78 ± 0.22 | 0.834 | 0.001* |
| Control | 1.45–2.07 | 1.83 ± 0.21 | |||
MDA, Malondialdehyde; TAC, total antioxidant capacity. *P<0.05 is significant. |
|||||
From the data in Table 5, it is clear that the mean values of hs-CRP, PCT, IL-18, ENA-78, and FCP were significantly higher in NEC group compared with those stated in the control group.
| Groups | Range | Mean ± SD | t-test | P | |
|---|---|---|---|---|---|
| hs-CRP (mg/L) | Patients | 4.6–6.9 | 5.99 ± 0.76 | 2.219 | 0.001* |
| Control | 0.8–2.5 | 1.27 ± 0.55 | |||
| PCT (ng/mL) | Patients | 5.2–52 | 18.5 ± 7.63 | 9.643 | 0.001* |
| Control | 0.5–4.9 | 1.98 ± 0.74 | |||
| IL-18 (pg/mL) | Patients | 90.33–111.12 | 98.84 ± 5.46 | 4.957 | 0.001* |
| Control | 35.07–49.71 | 42.16 ± 4.04 | |||
| ENA-78 (pg/mL) | Patients | 168.22–187.11 | 176.79 ± 5.66 | 3.234 | 0.001* |
| Control | 98.14–108.70 | 103.23 ± 4.98 | |||
| FCP (μg/g) | Patients | 222–597 | 399.8 ± 121 | 12.913 | 0.001* |
| Control | 5.8–101 | 38.4 ± 32.2 | |||
Hs-CRP, high sensitivity C-reactive protein; PCT, procalcitonin; IL-18, interleukin 18; ENA-78, epithelial neutrophil activating peptide-78; FCP, fecal calprotectin. *P<0.05 is significant. |
|||||
NEC is a critical disease of the digestive tract that affects preterm infants.20 Elevation of both chemokines and proinflammatory cytokines was reported in cases with NEC, which is a result of the reaction to the pathogenic microorganism and releasing various types of the inflammatory mediators according to the severity of infection.21
Our study showed significant elevated levels of both IL-18 and ENA-78 in NEC group when compared to the control group, which is in line with previous studies that documented that IL-18 induces the secretion of cytokines within neutrophils and promotes neutrophil accumulation22, and also high serum IL-18 measurement was present in the adult group which suffered from infections in another study work.23
In addition, Halpern et al.24 suggested that IL-18 had been proved to be essential and a crucial component in the mechanism of NEC development because the mice with low levels of IL-18 had very lower possibility of development of NEC. Concomitantly, several research works ensured the importance of ENA-78 in inflammatory diseases; for example, Keelan et al.25 reported elevated levels of ENA-78 in membrane extracts of preterm delivered pregnancies, which was similar to the pathogenesis of many forms of infections, inflammatory mediators, and cytokines liberation, such as TNFα, due to its importance to the host inflammatory reaction to microorganisms infection.
Another study confirmed that the main stimuli for rapid and early ENA-78 production are the early signals elicited during primary immune response as bacterial products, viral cause, and proinflammatory cytokines liberation.26
Moreover, sever inflammatory response and damage in the immature gut, secondary to abnormal intestinal bacterial colonization, play a detectable function in the NEC pathogenesis.27 Interestingly, in cases of various degrees of NEC, administration of agents, which either decrease ROS production or present as antioxidants, had been clearly done to decrease the tissue damage that ensures the function of ROS in NEC affection too.28
Furthermore, any redox imbalance caused by either excessive Reactive nitrogen species (ROS/RNS) release or limitation in antioxidant capacity results in mucosal injury, followed by necrosis owing to peroxidation of cellular membrane lipids29, which is also in line with our study as we documented significant elevated level of MDA in NEC group compared to the control group, while TAC level showed significant reduction in NEC group compared to the control group.
From another perspective, PCT and hs-CRP have been reported to be used in the diagnosis and prognosis of neonatal sepsis; however, the rise in their levels can be considered as reliable detectors of the early onset of NEC within the first 12 h.30
Our study agreed with the findings of another study suggesting that a negative PCT test may be used to exclude sepsis or NEC in neonates and limit hospital stay and the medication administration in neonates suspected for sepsis or NEC.31 Therefore, we should consider providing these easy, cheap, and early sensitive markers that were used in the early prediction of neonatal infection and NEC.32,33
Furthermore, this study showed that FCP level was increased in NEC cases, which is in agreement with the findings of Aydemir et al.34 who stated that the high levels of FCP could predict the occurrence of NEC. On the contrary, Selimoglu et al.35 documented that FCP did not have a function in the diagnosis of NEC, especially in the early NEC affection.
In our study, we documented the presence of positive correlation between IL-18, ENA-78, and MDA from one side and FCP from another side, suggesting the strong interrelation between cytokines and other groups of the markers of inflammation.
The clinical value and importance of our findings are that early prediction of neonatal NEC enables us for the early treatment which leads to better prognosis.
Our study has certain strengths. It was done on multiple biomarkers that help for early better prediction of neonatal NEC, and a group of doctors of biochemistry and clinical pathology were present in this study.
However, the study has certain limitations also. The number of patients in our study was low as these cases are considered rare in our unit.
The results of our study confirmed that the use of a combination of markers for early diagnosis of neonatal NEC clearly has great benefits in improving the early diagnostic performance of neonatal NEC as the clinical picture alone may not be enough to diagnose neonatal NEC, especially in early grades, which is valuable for the early prediction of NEC with early prevention, treatment, and better prognosis. FCP, ENA-78, and PCT can be considered as early markers for the diagnosis of NEC with early treatment and good prognosis.