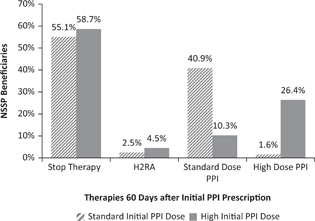
FIG 1. Percentage of NSSP Beneficiaries Prescribed Gastric Acid Suppression 60 Days after Initiation of Therapy.
Original Article
Jillian H. Carter1,2, Ingrid S. Sketris1,3, Hala Tamim4, Adrian R. Levy1 and Joanne M. Langley1,5,6
1Department of Community Health and Epidemiology, Dalhousie University, Halifax, NS, Canada
2Department of Emergency Medicine, Dalhousie University, Halifax, NS, Canada
3College of Pharmacy, Dalhousie University, Halifax, NS, Canada
4Faculty of Health, School of Kinesiology & Health Science, York University, Toronto, ON, Canada
5Canadian Center for Vaccinology, Dalhousie University, Halifax, NS, Canada
6Department of Pediatrics, Dalhousie University, Halifax, NS, Canada
Background
Proton pump inhibitors (PPIs) are often prescribed potentially inappropriately. The screening tool of older person’s potentially inappropriate prescriptions (STOPP) for therapeutic dose PPIs has been adapted to examine PPI discontinuation, dose reduction, or switching to Histamine-2 Receptor Antagonist (H2RA) after 60 days.
Objectives
The objectives of the present study were to (1) describe the use of acid suppression therapy (PPIs and H2RAs) 60 and 90 days after a new PPI dispensing, (2) assess predictors of lack of adherence to adapted STOPP criteria for PPI use, and (3) assess PPI dispensing over time.
Methods
This was a retrospective cohort study of beneficiaries of the Nova Scotia Seniors Pharmacare (NSSP) aged 66 years or older who were newly dispensed a PPI between January 1, 1997 and March 31, 2011. The main outcome measure was adherence to the adapted STOPP criteria, which was analyzed using logistic regression.
Results
A total of 14,453 participants were included: 89.8% beginning on standard dose and 10.2% beginning on high-dose PPI. Of those beginning on high-dose PPI, 26.4% were dispensed high-dose PPI at day 60 and 30.2% were dispensed high-dose PPI at day 90. Predictors of lack of adherence to our adapted STOPP criteria included age ≥86 years, rural residence, and hospitalization within 1 year prior to cohort entry.
Conclusions
Many PPI prescriptions dispensed for NSSP beneficiaries fail to adhere to the STOPP criteria. Predictors of lack of adherence to the adapted STOPP criteria were identified.
Proton pump inhibitors (PPIs) are among the most widely prescribed medications globally, most often for acid-peptic disorders. PPIs are ranked fourth in terms of spending by Canadian publicly funded drug programs for older (>65 years) adults.1 In 2015, 34.8% of Nova Scotia adults aged 65 and over using public drug plans were dispensed at least one prescription for a PPI.1
Use of PPIs has been rising worldwide.2 According to the Canadian Institute for Health Information (CIHI), 14% of adults over 65 years of age who were beneficiaries of publicly funded drug programs in 2002 had at least one claim per year for PPI therapy. This increased to 25% per year in 2009, then 27% per year in 2012, and 32% per year in 2016.3–5
The increase in PPI use may be due to policy, prescriber, or patient factors, including increased availability of both branded and generic PPIs. In Canada, each province’s drug insurance program has its own prescription drug formulary. When generic PPIs became available at decreased costs, some provinces reimbursed more types of PPIs or more indications for PPI therapy. This may have allowed more patients to access PPIs and more prescriptions to be reimbursed. At the prescriber level, there may have been an increase in the diagnosis of acid-peptic disorders, off label use, and reluctance to stop medications once started. Direct-to-consumer drug advertising (prohibited in Canada but available to the public via American media) may have led to increased patient demand for PPIs.2,6–8
Long-term use of PPIs leads to alterations in the gut microbiome and changes to gastric mucosa, which have been associated with negative outcomes especially harmful to the elderly (nutritional deficiencies, bone fractures, Clostridium difficile infection, etc.).9–30 Inappropriate prescribing of PPIs may increase the use of healthcare resources through increased drug costs and treatment of adverse drug events.31 Evidence from Canada and other countries suggests potentially inappropriate prescribing is present in 11–70% of patients prescribed PPI therapy.32–36 The Canadian Agency for Drugs and Technologies in Health’s (CADTH) 2007 Optimal Therapy report stated that up to 31% of Canadians received a double dose as initial PPI therapy for gastroesophageal reflux disease (GERD).37
One widely used tool to alert prescribers to potentially inappropriate prescribing in older adults is the screening tool of older person’s potentially inappropriate prescriptions and screening tool to alert doctors to right treatment (STOPP/START) criteria. This tool was developed using a Delphi process, validated in 2008, and updated in 2015 using a panel of 19 experts from 13 European countries.23,38 The STOPP/START criteria have been used internationally and have been shown to improve prescribing and patient outcomes.39,40 With regard to PPIs, the STOPP criteria state that potentially inappropriate prescribing may be present if “PPI for peptic ulcer disease at full therapeutic dosage [is given for greater than] eight weeks,” and if this is the case, “dose reduction or earlier discontinuation is indicated.”23 To date, studies examining adherence to STOPP criteria for PPIs have primarily been conducted in European countries, but work is ongoing in Canada.41–47
While the STOPP/START criteria for PPIs have not been previously implemented in Nova Scotia, other educational approaches to address potentially inappropriate prescribing have been implemented. In 2008 the Dalhousie Continuing Medical Education Academic Detailing Service sought to educate prescribers in Nova Scotia about comparative effective- ness of PPIs and Histamine-2 Receptor Antagonists (H2RAs), dosing, which patients are most likely to benefit, and adverse effects of PPIs.
The purpose of this study was to determine the concordance of PPI dispensations ‘to Nova Scotia Seniors Pharmacare (NSSP) beneficiaries with the 2008 STOPP criteria, the Dalhousie Continuing Medical Education Academic Detailing Service recommendations, and other clinical guidelines among NSSP beneficiaries. The specific objectives were to: (1) describe the use of PPIs and H2RAs 60 and 90 days after a new PPI dispensing between 1997 and 2011; (2) assess predictors of lack of adherence to adapted STOPP criteria regarding PPI use; and (3) describe PPI dispensing over time.
We conducted a retrospective cohort study using administrative data from Nova Scotia (population of 953,900 in 2017, with 19.8% being aged 65 and older).48
Ethics review was obtained from the Dalhousie University Health Sciences Research Ethics Board on July 24, 2013 (Reference number: 2013-3049).
Data were obtained from Health Data Nova Scotia (HDNS) at Dalhousie University.49 The analytic dataset was created by linking four databases: the NSSP Administrative Database, the Canadian Institute for Health Information’s Discharge Abstract Database, the Canadian Census Survey, and the Insured Patient Registry.
All Nova Scotians over 65 years of age without other drug coverage are eligible to receive drug insurance coverage from the NSSP program.50 Over 65% of eligible Nova Scotians enrolled in the program and subsequently received drug coverage through the NSSP in 2016.51 The NSSP database contains descriptive information about NSSP beneficiaries and is a record of the drugs dispensed and paid for by the NSSP. Centralized data on these dispensations include the age and sex of beneficiaries, as well as the anatomical therapeutic chemical (ATC; Appendix A) and type of acid suppression therapy used including dosage, duration, and dispensation date.
The Discharge Abstract Database was used to identify hospitalization at the time of cohort entry (one of the exclusion criteria). Urban/rural status of subjects was obtained from Statistics Canada and based on the forward sortation area (first three digits of the residential postal code).52 The Nova Scotia Medical Services Insurance (MSI) Insured Patient Registry was used to identify subjects who left Nova Scotia or died during the study period.
We required at least 1 year of baseline data for each subject before cohort entry such that the cohort consisted of subjects ≥66 years of age between January 1, 1997 and March 31, 2011. The sample included subjects who were newly dispensed PPI therapy (i.e. who had not been dispensed any PPI therapy in the 183 days prior to cohort entry). The subjects were followed for 90 days and were censored before this if they died or left the province.
For potentially eligible subjects, the first year data were used to apply exclusion criteria to create a cohort of subjects with uncomplicated gastric disease who were targeted by the STOPP criteria. We excluded subjects with medical conditions in the year prior to cohort entry for which PPI treatment could be indicated: Helicobacter pylori infection, gastrointestinal bleed, Barrett’s Esophagus, Zollinger Ellison syndrome, and cancer. We also excluded subjects who were dispensed H2RAs or non-steroidal anti-inflammatory drugs (NSAIDs) in the 6 months prior to cohort entry, as this may indicate baseline gastrointestinal disease. Patients were excluded if they were hospitalized at the time of cohort entry since we do not have detailed information about medication taken during hospitalization. Refer to Appendix B for a complete list of exclusion criteria and their justifications. Refer to Appendix C for a list of the diagnostic and procedure codes used to apply these exclusion criteria.
Since 1992, the NSSP required specific medical indications for reimbursement of PPIs (e.g., prophylaxis of NSAID-induced complications in patients who are at high risk for gastrointestinal bleeding). PPIs were reimbursed for 12 months, after which the prescriber was required to reassess the need for continued therapy. Some generic PPIs were launched on the market in Canada in 2005, and in January 2008 the NSSP program removed the reimbursement criteria on select PPIs; therefore, these were covered as full benefits when prescribed at standard daily doses. H2RAs have never had reimbursement criteria and were covered as full benefits. For PPI therapy, we determined the prescribed daily dose using the total quantity dispensed and the listed days’ supply. We defined standard and high PPI dosages as outlined by the Dalhousie Continuing Medical Education Academic Detailing Service; these are summarized in Appendix D.53
To determine concordance with the adapted STOPP criteria, we identified subjects receiving therapy longer than 60 days after initial PPI dispensing. We followed use at 90 days after initial dispensing as this was the maximum reimbursable supply for PPIs during the study period. We examined both PPI and H2RA use at 60 and 90 days after initial PPI dispensing to account for prescriptions with a 90-day supply. At day 60 or 90, there were four possible dispensing pattern outcomes: (1) remain on PPI therapy at the same dosage category (high vs. standard), (2) remain on PPI therapy but change the dosage category (high to standard vs. standard to high), (3) switch to H2RA therapy, or (4) stop acid suppression therapy.
Dispensing patterns at day 60 were determined by the dispensing closest and up to day 60. Patients who did not have a dispensation following day 0 and up to and including day 60 were categorized as having stopped therapy. Dispensing patterns at day 90 were defined the same way as dispensing patterns at day 60. Patients who did not have a dispensing following day 0 and up to and including day 90 were categorized as having stopped therapy. We did not examine dispensing beyond 90 days.
The 2008 STOPP criteria were the most recent version available to the physicians prescribing PPIs to subjects in our cohort. These guidelines state that patients with uncomplicated gastric disease should not use high-dose PPIs for longer than 60 days. This did not apply to severe GERD or peptic stricture requiring dilation or some types of severe esophagitis.23 We adapted this criterion to state that PPI therapy should not continue at any dose 60 days after being prescribed a high-dose PPI. We based this decision on several guidelines, including the Dalhousie Continuing Medical Education Academic Detailing Service document on PPIs and information from its authors, the CADTH Optimal Therapy Report on PPI prescribing, the Health Canada product monographs, Australian guidelines on PPI use, and the 2000 NICE guidelines.23,37,53–57 We also sought to identify subjects whose PPI therapy had not been reduced to H2RAs, which was one of the options suggested by the Dalhousie Continuing Medical Education Academic Detailing Service.53
All statistical analyses were conducted using SAS© software, version 9.4. Study population characteristics were summarized by frequency and proportion. The use of acid suppression therapy was described by percentage on days 60 and 90 after a new PPI dispensation.
To assess predictors of adherence to adapted STOPP criteria, only NSSP beneficiaries initially dispensed high-dose PPIs were considered. At days 60 and 90 there were two possible outcomes considered: remain on PPI therapy (at any dose) or stop PPI therapy (switch to H2RA therapy or stop all acid suppression therapy). Unadjusted odds ratios (ORs) with 95% confidence intervals (CIs) were calculated for each patient predictor at days 60 and 90. Multivariate logistic regression analysis was performed to assess the independent relationship between each patient predictor and outcomes at days 60 and 90. Patient predictors considered in the multivariate analysis included age, sex, urban/rural residence, and hospitalization in the year preceding cohort entry. The referent category for these analyses was stopping PPI therapy. Statistical significance was set at an alpha value of 0.05.
To describe PPI dispensing over time, the frequency of PPI dispensing was plotted over time. The proportion of high versus standard dose PPI initiation was also plotted over time.
A total of 14,453 subjects were included in the study cohort of NSSP beneficiaries newly dispensed PPIs, with the majority aged 66–75 years (Table 1). There were more women than men and 60% resided in urban areas. Roughly, one-third of subjects had been hospitalized in the year prior to cohort entry. The majority of subjects began PPI therapy at a standard dose (89.8%).
| Patients | |
|---|---|
| Patient Characteristics | Frequency n(%) |
| Age (years) | |
| 66–75 | 8,310(57.5) |
| 76–85 | 4,468(30.9) |
| 86+ | 1,675(11.6) |
| Sex | |
| Female | 8,845(61.2) |
| Male | 5,608(38.8) |
| Place1 | |
| Rural | 5,822(40.3) |
| Urban | 8,631(59.7) |
| Hospitalization in year prior to cohort entry | |
| No | 9,147(63.3) |
| Yes | 5,306(36.7) |
| PPI dose at cohort entry | |
| High | 1,475(10.2) |
| Standard | 12,977(89.8) |
| * Percentages may not sum to 100% due to missing values. High/standard-dose PPI therapy defined as: Esomeprazole (40 mg once daily/20 mg once daily), Lansoprazole (30 mg twice daily/30 mg once daily), Omeprazole (40 mg once daily/20 mg once daily), Pantoprazole (40 mg twice daily/40 mg once daily), Rabeprazole (20 mg twice daily/20 mg once daily). 1 Determined based on NSSP beneficiary postal code. |
|
Figure 1 summarizes acid suppression therapy dispensing patterns at day 60 compared to PPI therapy initiation. Over half (55.1%) of those beginning on standard dose PPI had stopped acid suppression therapy by day 60. A greater proportion (58.7%) of those beginning on high-dose PPI had stopped acid suppression therapy by day 60 compared to those beginning on standard dose therapy. Just under half (40.9%) of subjects beginning on standard-dose PPI remained on standard-dose PPI at day 60, whereas roughly one quarter (26.4%) of those beginning on high-dose PPI remained on high-dose PPI at day 60. A small proportion of subjects switched to H2RA therapy at day 60, although this was more common in those who began on high-dose PPI. Approximately 10% of those beginning on high-dose PPI switched to standard-dose PPI at day 60. Less than 2% of those beginning on standard-dose PPI switched to high dose PPI at day 60.

FIG 1. Percentage of NSSP Beneficiaries Prescribed Gastric Acid Suppression 60 Days after Initiation of Therapy.
Figure 2 summarizes acid suppression therapy dispensing patterns at day 90 as compared to PPI therapy initiation. Compared to dispensing patterns at day 60, fewer subjects stopped PPI therapy, more subjects stayed at their initial PPI dose, and more subjects switched to H2RA therapy. Subjects beginning on high-dose PPI switched to standard-dose PPI at day 90 (13.2%) more often than they did at day 60 (10.3%). Subjects who began on standard-dose PPI switched to high-dose PPI more often at day 90 (1.9%) than at day 60 (1.6%), although this still represented a very small proportion of those who began on standard-dose PPI.
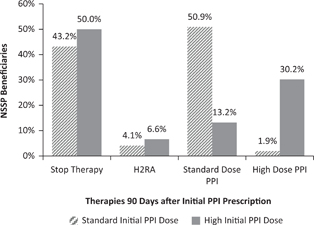
FIG 2. Percentage of NSSP Beneficiaries Prescribed Gastric Acid Suppression 90 Days after Initiation of Therapy.
The bivariate and multivariate logistic regressions were limited to subjects who began on high-dose PPI. Of these, 36.7% remained on PPI therapy at day 60 (Figure 1), which constituted a violation of the adapted STOPP criteria. All significant associations identified in the bivariate analysis were maintained in the multivariate analysis. Compared to subjects aged between 66 and 75 years, those aged 86 years or older were significantly more likely to continue PPI therapy at day 60 (adjusted OR = 2.4, 95% confidence interval [CI] [1.6–3.4]) and at day 90 (adjusted OR = 1.9, 95% CI [1.3–2.7]). Hospitalization within the year prior to cohort entry was significantly associated with continued PPI therapy at day 60 (adjusted OR = 1.4, 95% CI [1.1–1.7]) and at day 90 (adjusted OR = 1.2, 95% CI [1.0–1.5]). Subjects with rural postal codes were significantly more likely to continue PPI therapy at day 60 (adjusted OR = 1.3, 95% CI [1.1–1.6]) but not at day 90. Male subjects were significantly more likely than female subjects to continue PPI therapy at day 90 (adjusted OR = 1.2, 95% CI [1.0–1.6]) but not at day 60. The results of the logistic regressions are summarized in Table 2.
| Bivariate [OR (95% CI)] | Multivariate [OR (95% CI)] | |||
|---|---|---|---|---|
| 60 days | 90 days | 60 days | 90 days | |
| Age (years) | ||||
| 66–75 | 1 | 1 | 1 | 1 |
| 76–85 | 1.2 (0.9–1.5) | 1.2 (0.9–1.5) | 1.2 (0.9–1.5) | 1.2 (0.9–1.4) |
| 86+ | 2.3 (1.6–3.2)* | 1.8 (1.3–2.5)* | 2.4 (1.6–3.4)* | 1.9 (1.3–2.7)* |
| Sex | ||||
| Female | 1 | 1 | 1 | 1 |
| Male | 1.1 (0.9–1.4) | 1.2 (1.0–1.5)* | 1.2 (0.9–1.4) | 1.2 (1.0–1.6)* |
| Place | ||||
| Urban | 1 | 1 | 1 | 1 |
| Rural | 1.3 (1.1–1.6)* | 1.1 (0.9–1.4) | 1.3 (1.1–1.6)* | 1.1 (0.9–1.3) |
| Hospitalization 1 Year Prior to Cohort Entry | ||||
| No | 1 | 1 | 1 | 1 |
| Yes | 1.4 (1.1–1.7)* | 1.2 (1.0–1.5)* | 1.4 (1.1–1.7)* | 1.2 (1.0–1.5)* |
| * Statistical significance at α ≤ 0.05. | ||||
The frequency of PPI prescriptions dispensed to NSSP beneficiaries increased roughly tenfold over our observation period. The largest jump occurred between 2007 (approximately 800 PPI prescriptions dispensed) and 2008 (approximately 3,600 PPI prescriptions dispensed).
The proportions of high- versus standard-dose PPI initiation also changed between 2007 and 2008. Standard-dose PPI became proportionally more commonly dispensed, while high-dose PPI became proportionally less commonly dispensed (Figure 3).
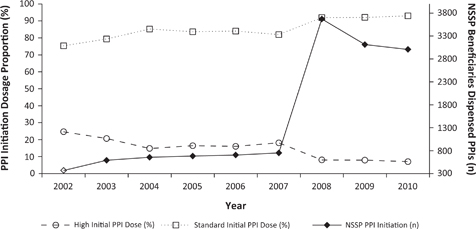
FIG 3. PPI Therapy Initiation in NSSP Beneficiaries between 2002 and 2010.
In this study, we examined potentially inappropriate prescribing of PPI usage in NSSP beneficiaries. PPI therapy was initiated at high dose in 10.2% of subjects. Of these, 36.7% continued on PPI therapy beyond 60 days, which represented potentially inappropriate prescribing according to our adapted STOPP criteria. Statistically significant predictors of potentially inappropriate prescribing after PPI initiation were age ≥86 years, hospitalization within 1 year prior to cohort entry, and rural residence.
The frequency of PPI dispensing to NSSP beneficiaries quadrupled between 2007 and 2008, with a proportional decrease in PPI initiation at high dosage. These changes coincided with the January 2008 removal of NSSP reimbursement criteria for two PPIs (rabeprazole and omeprazole).53 It was beyond the scope of this study to explore this further, but other factors may have influenced PPI dispensing over time. The changes in PPI dosing patterns seen in Figure 3 could be due to the Dalhousie Continuing Medical Education Academic Detailing Service or due to the increasing published literature related to the adverse effects of PPIs.58
Our study showed a lower rate of high-dose PPI therapy initiation than the national rate reported by CADTH in 2007 (31%).37 Our study showed more NSSP beneficiaries remaining on PPI therapy beyond 60 days (42.5% of those initiated at standard dose, 36.7% of those initiated at high dose) compared to similar studies of Northern Ireland populations (27.9–38.9%).41,43
Previous literature using the STOPP criteria has yielded conflicting results on the relationship between older age and potentially inappropriate prescribing of PPIs.41,43,44 Hospitalization has not been commonly studied as a predictor of potentially inappropriate prescribing of PPIs; however, a Canadian study at the Vancouver General Hospital found that 36% of inpatients were ordered a proton pump inhibitor without an appropriate indication.59 The association between hospitalization and PPI therapy seen in this population could be related to comorbidity leading to polypharmacy, which has been found to be predictive of potentially inappropriate prescribing of PPIs in multiple studies.41,43,44 The effect of rural residency on PPI use has not been commonly studied. The relationship seen in this study could be explained by the scarcity of rural doctors in Nova Scotia and the long distances travelled by rural residents to see their doctor. This may motivate physicians to decrease the visit burden by giving the maximum supply reimbursable by NSSP (90 days).
This was the first time adherence to STOPP criteria for PPI was explored in Nova Scotia using a large population database. Our study shows there could be a role for further guidance on PPI dose and duration. Specifically, efforts should be focused toward patients aged 86 years and older, those recently hospitalized, and those residing in rural areas. Given the large proportion of Nova Scotians on a publicly funded drug plan being dispensed PPIs, implementation of the adapted STOPP criteria represents an opportunity for decreased patient harm and wise use of public resources. A cost utility analysis by Moriarty et al. (2019) revealed interventions to deprescribe PPIs would be cost-effective when weighed against adverse effects (hip fracture and C. difficile infection) of maximal dose PPI use beyond 8 weeks.31 This is congruent with the position of Choosing Wisely Canada, which advises patients and doctors to avoid long-term PPI therapy without an attempt to stop/reduce PPI at least once yearly.60 Application of the STOPP criteria for PPIs could be facilitated through electronic health record and administrative data assessment to provide feedback to physicians and healthcare organizations.61–63
There were several advantages of this study. The use of longitudinal administrative data allowed for a large population-based sample that captured the majority of PPI use in our cohort.64 Our population included urban and rural older adults residing in nursing homes as well as community dwellings. Our exclusion criteria made the STOPP criteria more directly applicable to our cohort as we excluded those requiring long-term acid suppression therapy by excluding PPI use up to 1 year prior to cohort entry. In addition, we describe the context related to educational and policy initiatives that occurred in Nova Scotia, which may have impacted prescription patterns.
This study was not without limitations. Our data are older and may not reflect current prescribing practices. In Canada, omeprazole 20 mg (and salts) became available without a prescription from September 17, 2014 and esomeprazole 20 mg (and salts) became available without a prescription from August 18, 2016.64–66 Other PPIs still require a prescription in Canada. The data were collected prior to these changes and therefore may capture a greater proportion of PPI use than data collected after these PPIs became available without a prescription. Our data provide a baseline for future educational interventions and benchmark for other jurisdictions.
Our sample was limited to NSSP beneficiaries, which constituted just over 65% of Nova Scotians in this age cohort, the remainder of which may have different PPI use patterns.51 The dataset measured dispensing only; therefore, there was no information on prescribing by healthcare professionals or usage by patients. Nor were we able to determine indication for therapy or severity of symptoms on therapy initiation. We could not determine all concurrent drug therapy or concomitant diseases.
Our exclusion criteria did not eliminate all potential long-term users of PPI therapy, such as patients with erosive gastritis, esophageal strictures/dilations/varices, patients on anticoagulants, and patients receiving palliative care. We did not identify if patients began PPI therapy in hospital and these patients may have more complex indications for PPI use. We suspect that the proportion of PPI therapy initiations occurring in hospital is small and therefore the impact of excluding these patients is likely small. This is supported by a previous study examining medication adherence in patients with another common illness (diabetes), which revealed that the majority of seniors initiating medications for diabetes were not hospitalized.67 This study looked at only basic predictors of PPI usage and did not adjust for the potential confounding bias in smoking status, diet or lifestyle factors, prescriber characteristics, chronic comorbid diseases, total number of medications per patient, use of those H2RAs obtained without a prescription, or ulcer causing drugs (e.g., corticosteroids). The Charlson Comorbidity Index could not be applied to this study due to these data being unavailable in our cohort.68 We were also not able to determine patient symptoms or quality of life outcomes while receiving PPIs or on discontinuation of PPI therapy. We did not have information on prescriber characteristics such as university for medical education, years in practice, professional development activities, or practice characteristics such as solo versus group practice, etc.
In conclusion, we used population-based data to explore PPI usage in NSSP beneficiaries. There was an overall increase in PPI dispensations in this population during the study period. The majority of NSSP beneficiaries beginning PPI therapy did so on a standard initial dose, but many NSSP beneficiaries remained on PPI therapy longer than 60 days. These patients may benefit from dose reduction or limiting PPI use to on-demand therapy.69 The factors age ≥86, rural residency, and hospitalization within the year prior to cohort entry were identified as predictors of potentially inappropriate prescribing after PPI initiation at high dosage. Through this study, we raise awareness for the STOPP criteria (developed in Europe for an international audience) and show clinicians how we and others have used the criteria to document areas of improvement for patient care.
Future research could seek to explore more predictors for potentially inappropriate prescribing of PPIs and use both qualitative and quantitative research methods to determine reasons for lack of adherence to the guidelines at the patient, prescriber, and health system levels. Future research could also examine the effects of the removal of the NSSP reimbursement criteria for rabeprazole and omeprazole over time using time series analysis. Investigation of the effects of over-the-counter PPI use on dispensations of prescribed PPIs to patients in Canada may be beneficial. The implications of increased prescribing of PPIs seen in this study could be further examined with regard to the incidence of PPI-associated health risks over time. Moreover, designing and evaluating potential interventions such as incorporating PPI STOPP criteria into computerized health record decision-making could improve adherence to the STOPP criteria.
The data used in this report were made available by Health Data Nova Scotia of Dalhousie University. Although this research is based on data obtained from the Nova Scotia Department of Health and Wellness, the observations and opinions expressed are those of the authors and do not represent those of either Health Data Nova Scotia or the Department of Health and Wellness. Funding was provided by the Nova Scotia Health Research Foundation (grant number: PSO-SSG-2011-7596). Resources and support for this study were also provided through the Canadian Network for Observational Drug Effect Studies.
The authors have no financial or other affiliations that would represent a conflict of interest regarding this study.
PPIs
Dexlansoprazole—A02BC06
Esomeprazole—A02BC05
Lansoprazole—A02BC03
Omeprazole—A02BC01
Pantoprazole—A02BC02 *Not classified as magnesium or sodium
Rabeprazole—A02BC04
H2RAs
Cimetidine—A02BA01
Famotidine—A02BA03
Nizatidine—A02BA04
Ranitidine—A02BA02
Ranitidine bismuth citrate—A02BA07
NSAID
Indomethacin—S01BC01
Oxyphenbutazone—S01BC02
Diclofenac—S01BC03
Flurbiprofen—S01BC04
Ketorolac—S01BC05
Piroxicam—S01BC06
Bendazac—S01BC07
Salicylic acid—S01BC08
Pranoprofen—S01BC09
Nepafenac—S01BC10
Bromfenac—S01B
The following classifications were used to identify NSSP beneficiaries who met our exclusion criteria:
The following are specific exclusion criteria and the codes used to identify them:
| PPI Therapy | ||
|---|---|---|
| Drug | Standard Dose | High Dose |
| Esomeprazole | 20 mg once daily | 40 mg over 24 hours |
| Lansoprazole | 30 mg once daily | 60 mg over 24 hours |
| Omeprazole | 20 mg once daily | 40 mg over 24 hours |
| Pantoprazole | 40 mg once daily | 80 mg over 24 hours |
| Rabeprazole | 20 mg once daily | 40 mg over 24 hours |