INTRODUCTION
The alveolar cleft is a common congenital deformity resulting from faulty primary palate development between 4 and 12 weeks of gestation.1 The alveolar defect occurs as a gap that widens from bottom to top, with the piriform aperture revealing the biggest bony gap and giving the appearance of a tornado.2 The most typical alveolar deformity is seen between the upper lateral incisor and the canine. Lexer 3 was the first to describe a nonvascular bone graft to reconstruct the cleft in the maxilla. Gingivoperiosteoplasty is a boneless, bone transplant method created by Skoog.4 Alveolar cleft graft has been surrounded by controversy concerning the best grafting material and the timing of treatment. The timing of bone grafting is generally categorized as “primary,” “secondary,” and “delayed.”5 The primary bone graft (PBG) utilizes rib bone to reconstruct the alveolar cleft at the infant stage.6 On closer inspection and follow-up, the side effects of PBG have been documented, including midface retrusion and anterior crossbite. Secondary alveolar bone grafting (SABG) has been found to be most effective between the ages of 6 and 12 years in majority of the patients. The eruption of the canine or lateral incisor determines the timing for SABG. The bone transplantation will be completed before the emergence of the lateral incisor or canine root into the cleft site and at half-to-two-thirds of their development.7
When a patient is above 14 years old and had missed the treatment at the recommended time, delayed or late secondary bone-grafting is performed. Improved lip support and nasolabial angle may be beneficial for older patients. However, the main drawback of this approach is that the crestal height returns to preoperative levels, and the periodontal and alveolar support for the teeth next to the cleft does not improve considerably.8
Therefore, the main goal of the SABG is to provide enough bone and periodontal support to teeth that are erupting through or located near to the cleft area, so that they can be moved to the grafted area with orthodontics. Other advantages of this method include stabilizing the premaxilla in individuals with bilateral clefts, closing the oronasal fistula, and supporting the nasal base.7 Alveolar cleft repair has involved the use of numerous autologous, alloplastic, and xenogeneic bone components as well as growth factors. The best bone graft source among these is autologous cancellous bone.9 Alloplastic bone grafts composed mainly of hydroxyapatite (HA) and β-tricalcium phosphate (β-TCP) with osteoconductive properties are allocated to the mineralized collagen structure, which provide a scaffold that facilitates bone deposition.10 Many study reports have shown that the use of alloplastic grafting materials decreased host incompatibility, infection rates, and morbidities at the donor site.11 The hydroxyapatite/tricalcium phosphate biomaterial (QualyBone BCP®, QualyLive, Amadora, Portugal) is a synthetic ceramic made of 75% HA and 25% TCP. It possesses macroporous property that promotes neovascularization and bone cell proliferation in voids.12
Platelet-rich plasma and platelet-rich fibrin (PRF) are two substances that have been created and utilized to speed up bone healing, promote bone formation, and lessen bone resorption.13 Choukroun et al.14 first described PRF as a fibrin matrix that contains many growth factors, cytokines, and other cells that enhance tissue healing and bone regeneration.15 High numbers of host immune cells are present in PRF, a unique formulation that is entirely autologous, created without the use of anticoagulants.16 The purpose of this study was to assess the effect of PRF on bone healing and the amount of bone formation 6 months following surgery.
PATIENT AND METHODS
The study included 20 patients (14 men and 6 women) with congenital unilateral alveolar clefts. Of these, 13 cases had alveolar cleft on the left side, while the remaining 7 cases had a right-sided alveolar cleft. The patients were between the ages of 7 and 13 years. All cases were operated at the Oral and Maxillofacial Department, at the Al-Wasity Teaching Hospital, Baghdad, Iraq, from November 2020 to June 2022. The selection of patients was based on the following criteria: (1) non-syndromic congenital alveolar cleft, (2) good oral hygiene, (3) absence of any systemic diseases or bleeding disorders, (4) no previous attempts of bone grafting at the cleft site and (5) full information regarding the procedure was provided to the patients’ parents, and parental consent was obtained before the surgery. Any patients outside these criteria were excluded from the study. Randomly, two equal groups of patients were created; (1) Group I contained 10 patients, who underwent reconstruction of the alveolar cleft by using the alloplastic bone substitute (QualyBone BCP®, QualyLive, Amadora, Portugal) with PRF, and (2) Group II contained 10 patients, who underwent grafting with an alloplastic bone substitute (QualyBone BCP®, QualyLive, Amadora, Portugal) only.
To rule out any bleeding problems or systemic diseases that could affect the operation procedure, patients were sent for blood investigations and an electrocardiogram before surgery. Also, all patients underwent cone-beam computed tomography (CBCT) at the radiology department preoperatively, to determine the side and size of the alveolar cleft defect and the relation of adjacent teeth to the cleft area.
SURGICAL PROCEDURE
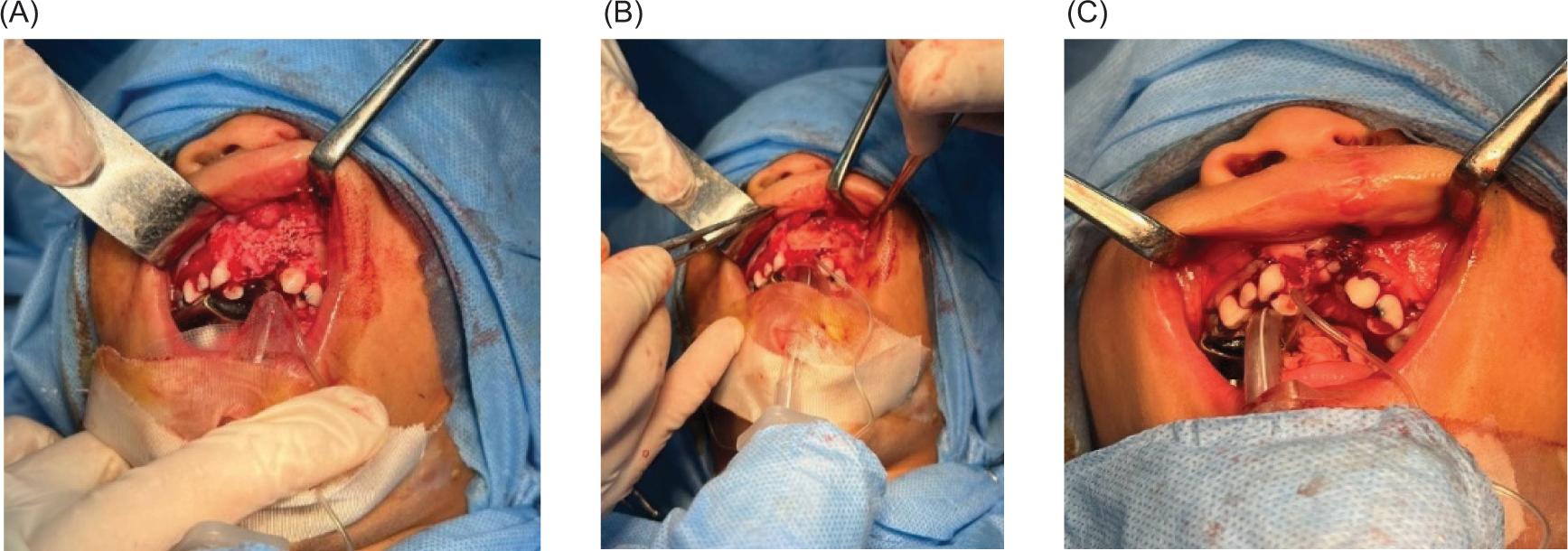
Oral intubation was performed under general anesthesia for all procedures (Figure 1A). Local anesthesia (lidocaine) was used to infiltrate the surgical site. A buccal mucoperiosteal flap was reflected from the canine tooth on the non-cleft side, and around the cleft deformity to the distal surface of the molar tooth on the cleft side. Palatal mucoperiosteal flaps were reflected and resutured to reconstruct the palatal fistula, if present. After that, the nasal mucosa was separated from the oral mucosa (Figure 1B) by fine dissections, and the nasal mucosa was sutured by using vicryl 0/4. At this step, in Group I, an alloplastic bone substitute was placed in the metal jar, while 20 ml of venous blood was aspirated from the patient to prepare the PRF membrane. The PRF tube was centrifuged (Wotefusi, Shanghai, China) immediately (within 2 min) after aspiration at 2700 revolutions per minute (rpm) for 12 min, and the sample was separated into three layers (Figure 2A). Poor-platelet plasma was obtained in the upper layer, fibrin clot was seen in the intermediate layer, and the lower layer contained red blood cells. The fibrin clot from the middle layer was taken and compressed using a metal PRF box to obtain the PRF membrane (Figures 2BC). Bone substitute was placed over the alveolar defect, and the PRF membrane adapted over the bone substitute (Figures 3AB). While in Group II, the bone substitute was applied directly to the alveolar defect without PRF. Finally, the oral mucosa was closed by interrupted 3-0 silk sutures in a tension-free manner in both groups (Figure 3C).
FIG 1. (A) An intraoperative image showing the cleft side and intraoral intubation. (B) Buccal mucoperiosteal flap reflected and separated the nasal mucosa from the oral mucosa.
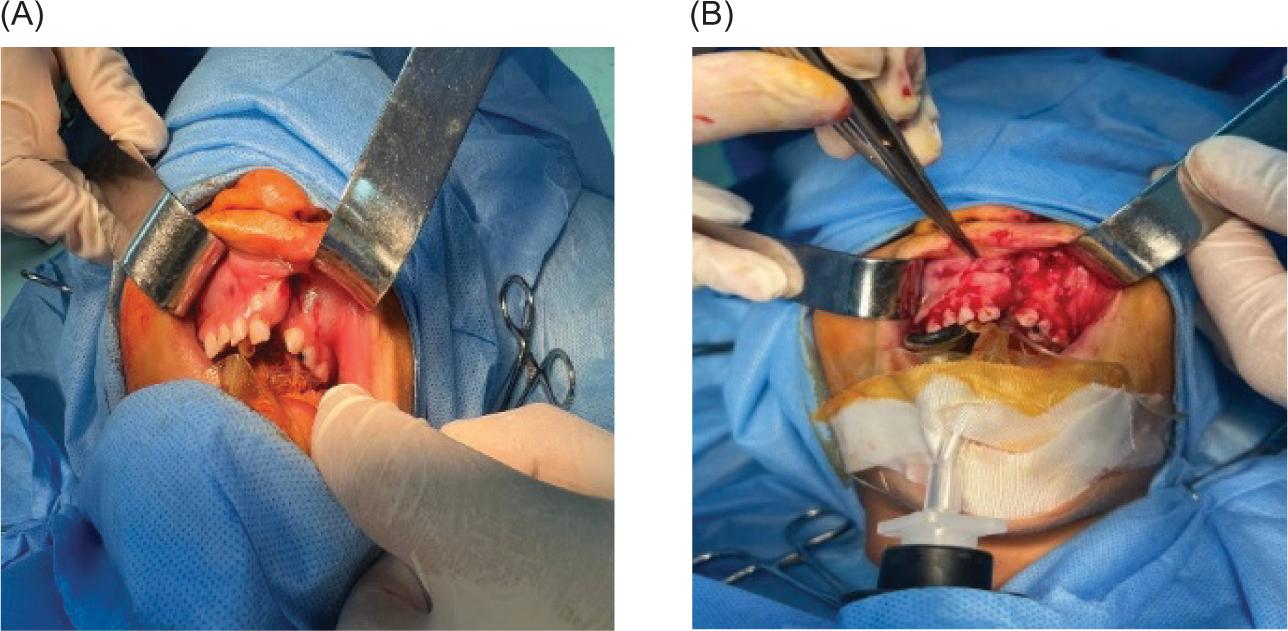
FIG 2. (A) Platelet-rich fibrin (PRF) tube showing the three layers after centrifugation; (B) Fibrin clot taken from the middle layer of the tube; (C) PRF membrane obtained by using the metal PRF box.
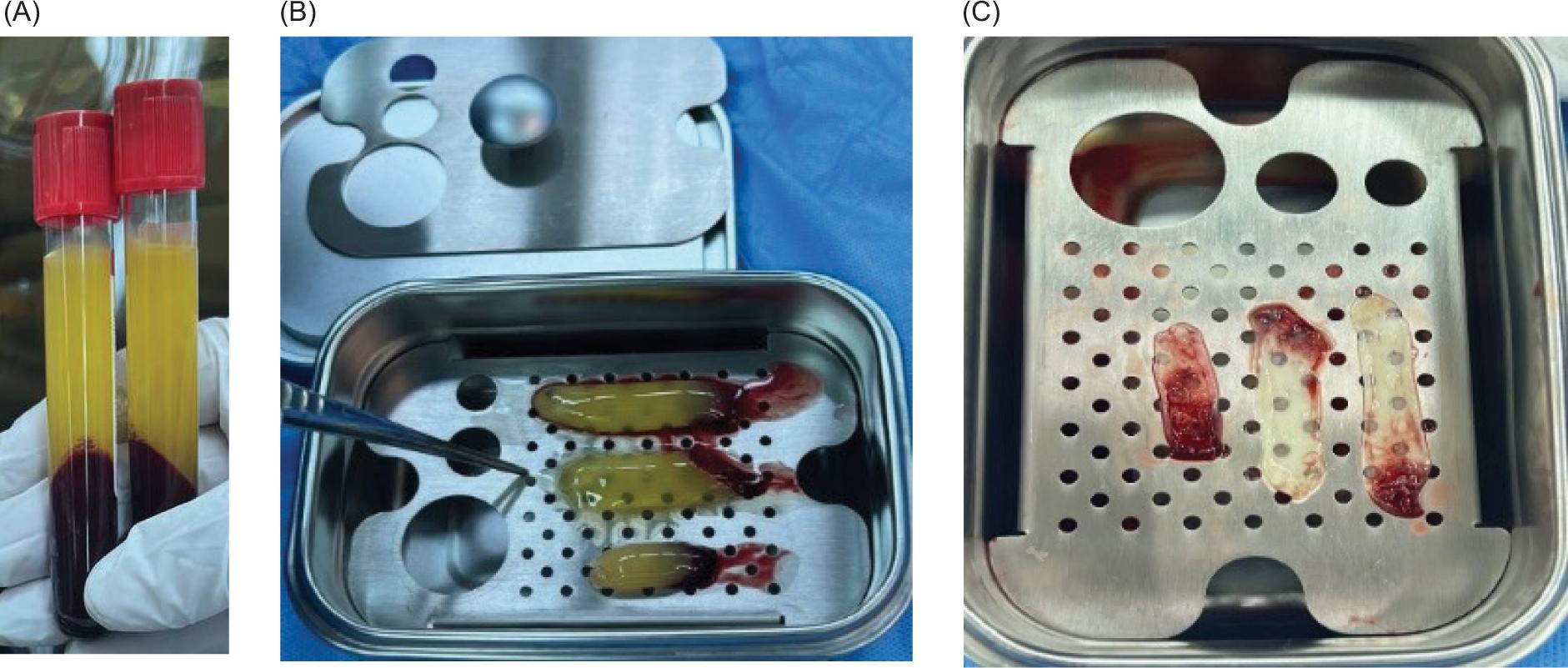
FIG 3. (A) Bone substitute placed over the alveolar defect; (B) Platelet-rich fibrin (PRF) membrane adapted over bone substitute; (C) Closure of oral mucosa in a tension-free manner.
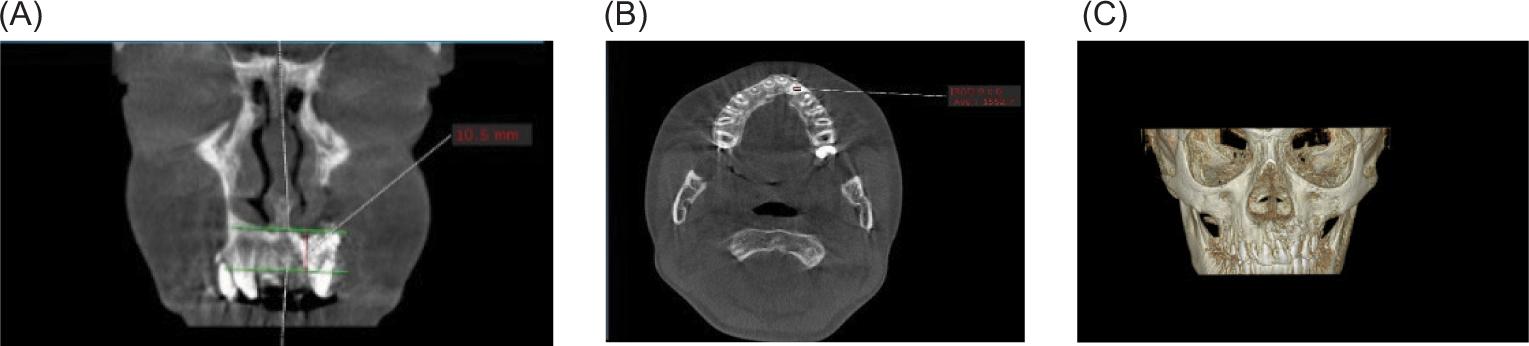
Postoperatively, the range of patient-stay in the hospital was from 1 to 2 days. Patients were instructed to remain on a fluid diet for the first 5–7 days. Antibiotics, analgesics, and mouthwash were prescribed for all patients postoperatively. Around 7–10 days after surgery, the intraoral sutures were removed. All patients in both groups underwent CBCT 6 months after the surgery to evaluate the height, bone density, and bony bridge formation at the alveolar defect. The results obtained from the two groups were compared to evaluate the effect of PRF added with bone substitute on the bone healing process. The CBCT coronal view was used to measure the height of bone formation in the 6 months after surgery for both groups. This was achieved by drawing two parallel lines, one extending from the piriform apparatus on the non-cleft side to the reconstructed piriform area on the cleft side, and the other drawn at the level of the cementoenamel junction of the central incisor, and measuring the distance between them, which represented the height of bone formation at the cleft side (Figure 4A). The axial view was used to assess the bone substitute density in the densest region in both groups 6 months after surgery by using the Hounsfield Scale (Figure 4B). The 3D view was used to assess the continuity of the alveolar bone 6 months after surgery (Figure 4C).
FIG 4. (A) Six months postoperatively, cone-beam computed tomography (CBCT) coronal view revealed bone height at the alveolar defect by drawing two parallel horizontal lines, one at the level of piriform fossa and the other at the level of the cementoenamel junction of adjacent teeth; (B) CBCT axial view used to measure bone density at the densest area 6 months postoperatively; (C) The 3D view revealed bone continuity and formation at the alveolar site.
RESULTS
A total of 20 patients with non-syndromic congenital unilateral alveolar cleft were recruited to this study. All of them were operated under general anesthesia to reconstruct the alveolar defect, and they were randomly allocated to close the defect by alloplastic bone substitute with PRF (Group I, included 10 patients) or by alloplastic bone substitute only (Group II, included 10 patients). The patients’ ages varied from 7 to 13 years, their mean age was 9 years, and their standard deviation (SD) was ±1.81. Regarding gender, the proportion of males was higher than females (70% vs 30%), while 65% of the study patients were diagnosed with a left-sided cleft. Abnormal bleeding was not observed in any patients. No evidence of infection was observed in any patients, except two cases of wound dehiscence observed in Group II after 3 days of operation, which were treated by irrigation and resuturing, and patients were also instructed on good oral hygiene practices.
Radiographic CBCT was done at 6 months postoperatively to measure the bone height, density, and alveolar ridge continuity. After 6 months following surgery, bone continuity was seen in all patients in both groups, but was more obvious in Group I.
After 6 months, the mean values of bone density were 807.06 HU and 686.32 HU for Groups I and II, respectively. Consequently, there was a very significant difference between the two groups (P = 0.001), which clearly showed that bone formation was significantly denser in Group I than in Group II during the same interval (Table 1).
TABLE 1. Comparison of the mean values of bone density, 6 months postoperatively.
| Group | Mean value + SD of bone density 6 months postoperatively | P |
|---|---|---|
| Group I | 807.06 HU ± 66.2 HU | 0.001 |
| Group II | 686.32 HU ± 63.7 HU |
SD, standard deviation.
In addition, 6 months post operation, the mean value of bone height for Group I was 8.74 mm, while the mean value of Group II was 6.94 mm. Consequently, there was a significant difference between the two groups (P = 0.001). This clearly showed that new bone formation was significantly higher in Group I than in Group II during the same time periods (Table 2).
TABLE 2. Comparison of the mean values of bone height, 6 months postoperatively.
| Group | Mean value + SD of bone height 6 months postoperatively | P |
|---|---|---|
| Group I | 8.74 mm ± 0.73 mm | 0.001 |
| Group II | 6.94 mm ± 0.92 mm |
SD, standard deviation.
DISCUSSION
There have been controversies on the time of surgical intervention and the materials used in the management of the alveolar cleft since the first documented series of bone grafts in 1955. Different grafting materials are used in alveolar cleft reconstruction process such as autogenous, allogenic, alloplastic, or tissue engineered materials.17 The autogenous iliac bone graft is considered a golden material for the surgical treatment of alveolar cleft. However, autogenous grafts have a number of drawbacks, including the need for a second surgical site, higher surgical costs, the possibility of scarring at the donor site, and higher surgical risks, such as excessive bleeding, infection, inflammation, and pain.18 The male to female ratio in this study was 2.3:1, with 14 males (70%) and 6 females (30%). The literature has revealed that cleft lip and palate is more frequent in males than in females, while cleft palate is more prevalent in females.19 The alloplastic materials have many advantages, including their greater availability, nonrequirement of a donor site, and the lack of disease transmission.20
This study used an alloplastic bone substitute (QualyBone BCP®, QualyLive) to reconstruct the congenital alveolar cleft. The Qualybone BCP is composed of 75% HA and 25% tricalcium phosphate (β-TCP) and is reabsorbed between 6 and 24 months. It is a 100% synthetic, porous ceramic. The primary goal of this biomaterial is to fix bone voids or defects to make the dough formation physiologically suitable. Due to its mesh-like construction, which has a high porosity, the bone cells can grow more quickly into open areas. The excellent opacity properties enabled radiographic monitoring of bone regeneration.
In this study, 6 months after reconstructions, the density and bone height at the cleft site were compared when grafted with an alloplastic bone substitute and PRF (Group I) versus when grafted with only an alloplastic bone substitute (Group II). This comparison was made to assess the impact of PRF on the bone healing process. The most significant growth factors of PRF are transforming growth factor, platelet-derived growth factor, insulin-like growth factor 1, vascular endothelial growth factor, and epidermal growth factor. The PRF also contains immune cytokines such as interleukins (IL-1β, IL-4, IL-6) and tumor necrosis factor. All these factors have an obvious effect on bone regeneration and formation.21
In this study, CBCT measurement at 6 months postoperatively revealed the formation of a bony bridge at the cleft site in both groups, which was more obvious in Group I. There was also a significant increase in the height and bone density in Group I when compared with Group II. These results were in agreement with the findings of Simonpieri et al.,22 Karayürek et al.,23 and Nacopoulos et al.24 They investigated the effectiveness of PRF on bone development and concluded that the combined use of PRF and β -TCP helped increase bone regeneration and decrease bone resorptions. The time of edema was also decreased, and an eventful soft tissue healing was observed in Group I. While in Group II, there were two incidences of wound dehiscence and an increased length of time of edema was observed. The lack of PRF in Group II was the possible reason for this. In addition to protection of the surgical site, PRF membranes also aid in hemostasis, promotes the repair of soft tissues, control inflammation, and quicken the healing process.
CONCLUSIONS
Numerous growth factors and cells, and a fibrin matrix found in PRF have been proven to have an impact on the vascularization and regeneration of bony defects. Our study found that the use of PRF in combination with an alloplastic bone substitute maintained the height of the graft, kept the particles connected, and had a significant effect on the density of the alveolar graft after 6 months post operation.