INTRODUCTION
Leishmania is a parasite belonging to the family Trypanosomatidae, which causes an infectious disease called leishmaniasis. The main mode of transmission is through a bite from a sand-fly. There are three types of leishmaniasis: (i) cutaneous, (ii) mucocutaneous, and (iii) visceral leishmaniasis (kala-azar).1,2 The clinical manifestations differ according to the type of leishmaniasis: (i) localized cutaneous leishmaniasis (LCL) is presented with an ulcerative skin lesion at the site of the infection; (ii) diffuse cutaneous leishmaniasis (DCL) displays multiple nodules (non-ulcerative); (iii) mucosal leishmaniasis is presented with destructive mucosal inflammation; and (iv) visceral leishmaniasis.2
Leishmaniasis was observed in all regions excluding Oceania, and it was endemic in limited geographical regions such as in Middle East, Northeastern Africa, Southeastern Mexico, Central and South America, and Southern Europe.3 Zoonotic transmission is the main mode in cutaneous leishmaniasis (CL). However, leishmaniasis caused by Leishmania tropica, are mainly anthroponotic. The only confirmed vectors of human transmission were through sand-flies.4 The most widely spread leishmaniasis is the CL, where one-third of the CL cases were confined in the area between Middle East and Central Asia. In addition, it was also observed in the Mediterranean and the United States. Most of the confirmed cases were also found in Brazil, Afghanistan, Iran, Algeria, Syria, Colombia, North Sudan, Ethiopia, Peru, and Costa Rica. Globally; they account for about 75% of the estimated CL incidence.5
The clinical features may vary depending on the characteristics of the parasite and on the effectiveness of the host immune response. CL are either LCL (restricted to a distinct part of the skin, usually single part) or DCL (multiple lesions). LCL is mostly observed in body parts that are exposed to insect bites. The most susceptible regions are the ears followed by other parts such as upper lip, nose, cheeks, hands and forearm, legs, and ankles.6 The average incubation period is 1–4 weeks and in certain cases, it can last for up to several years.
LCL is characterized by swelling and locally increased temperature. An erythematous papule is observed at the site of bite and may be accompanied by pruritus. The size of the papule ranges from 1 to 10 mm in diameter, which changes into a vesicle within 2 days and then into a pustule and finally this results in an ulcer, which is round-shaped with nodular or thick borders.6 DCL is characterized by the absence of cellular immune response to the antigens of the parasite. This will allow dissemination and the lesions will start developing in most parts of the skin, excluding the scalp.7,8
The World Health Organization (WHO) has recommended systemic or intralesional antimonials (meglumine antimoniate or sodium stibogluconate) for CL treatment. The use of antimonials is the only effective treatment that provides satisfactory results to various clinical forms of leishmaniasis.2 Recently, intralesional treatment was documented as the primary treatment for LCL caused by Leishmania tropica, Leishmania major, and Leishmania panamensis. Intralesional treatment causes damage to the cellular membrane and destroys the microtubules that prevent organism division. For CL, antimonials are the mainstay therapy that is administered intralesionally (1–5 mL per session every 3–7 days) in addition to systemically (20 mg/kg for 20 days).9
Fluoroquinolines are an interesting class of antibiotics, they are used to treat bacterial infections and also exerts, antifungal, antiviral, and antiparasitic actions.10 Levofloxacin (Levaquin) is a fluoroquinolone antibacterial agent that is an L-isomer of the racemate ofloxacin. It inhibits DNA gyrase and topoisomerase IV in bacteria. Levofloxacin has a wide range of action against Gram-positive and Gram-negative aerobic bacteria as well as atypical bacteria, but has poor activity against the majority of anaerobic bacteria.
In this study, intralesional levofloxacin were used for the treatment of LCL to investigate its effectiveness as there is low availability sodium stibogluconate with high cost.
METHODS
Patients and Clinical Evaluation
A cohort study was conducted from November 2021 to March 2022 at dermatologist clinic in Badrah district of Wasit province, in the east of Iraq. Fifty participants (28 male and 22 female) with age ranging from 8 to 34 (median ± SD; 20 ± 6.58) with LCL were enrolled in this study. The diagnosis of LCL was performed at the clinic. Patients with diabetes mellitus and previous diagnosis of CL were excluded from the study. This work was performed according to the Helsinki 11 declaration and all the participants, parents, or legal guardians of the studied individuals were informed about the purpose and the expected benefits of this study before agreement of participation was documented. Patients with volcano-shaped ulcer with lesion diameter ranging from 10 to 30 mm (median ± SD; 20 ± 4.93) and having horizontal or deep vertical pain on examination, tenderness, or occasional itch were enrolled to the current work.11
Treatment
Drug therapy was performed to all the patients then they were followed-up in-clinic. The amount of dose decided was increased over time according to the response, depth, horizontal size, ulcer diameter, duration, number of boils, the hostility of the disease. The average dose was 1 mL of Levofloxacin solution was administered for every 1 cm diameter of boil and this dose is administered every 5 days with an average of three to five times during the treatment period.
Criterion of Cure, Failure
It is considered to be cured only after the complete healing of LCL after clinical examination until 1 month of the treatment. However, lack of lesion improvement after therapy is considered failure. The improved lesions without complete healing were considered failure.
Statistical Analysis
The data of the study were stored in Microsoft excel spreadsheet and analyzed using SPSS software 20 and Microsoft excel program (2010). Numeric variables were expressed as mean ± SD and all statistical comparisons were performed using means of independent t-test and P ≤ 0.05 was considered statistically significant. The correlation was performed between all the parameters using Pearson correlation test.12
RESULTS
Results illustrated in the present work revealed that there were non-significant differences between male and female in age, diameter of lesion before and after receiving treatment, healing time, and number of visits required to achieve the intended outcome as illustrated in Table 1.
TablE 1. The differences between male and female patients in age, initial lesion diameter, final lesion diameter, time for healing, and number of visits.
| Parameters | Gender | N | Mean | Median | Std. deviation (SD) | Std. error (SE) | P |
|---|---|---|---|---|---|---|---|
| Age | Male | 28 | 20.64 | 20 | 6.31 | 1.19 | 0.635 |
| Female | 22 | 21.54 | 20 | 7.02 | 1.5 | ||
| Initial lesion diameter | Male | 28 | 20.04 | 20.5 | 4.68 | 0.88 | 0.955 |
| Female | 22 | 19.95 | 19 | 5.34 | 1.14 | ||
| Final lesion diameter | Male | 28 | 15 | 15 | 3.53 | 0.67 | 0.767 |
| Female | 22 | 14.68 | 14 | 4.02 | 0.86 | ||
| Time for healing (day) | Male | 28 | 22 | 22.5 | 4.12 | 0.78 | 0.609 |
| Female | 22 | 22.59 | 23 | 3.9 | 0.83 | ||
| Number of visits | Male | 28 | 3.75 | 4 | 0.7 | 0.13 | 0.336 |
| Female | 22 | 3.95 | 4 | 0.79 | 0.17 |
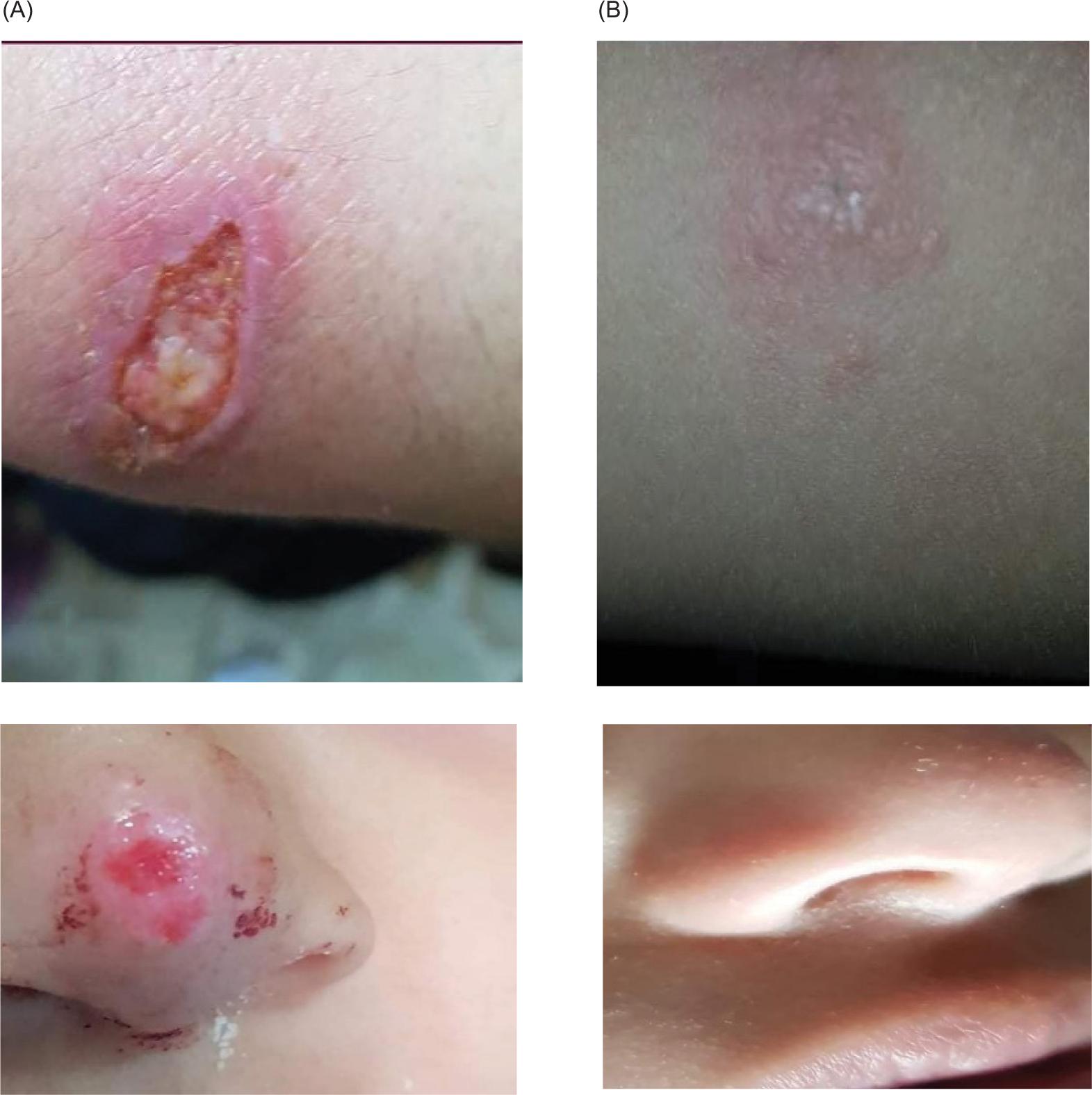
As obtained in the present study, 45 out of 50 patients who received intralesional levofloxacin showed response toward treatment and were healed completely (cure rate of 90%). Some of the LCL cases that were cured and after intralesional levofloxacin treatment are shown in Figure 1.
FIG. 1. (A) LCL before the treatment. (B) LCL after the treatment with intralesional levofloxacin.
Statistically, results illustrated in the Table 2 revealed that the lesion diameter of the LCL were reduced significantly (p < 0.001) after receiving the intralesional treatment with levofloxacin from (median ± SD; 20 ± 4.93) to (median ± SD; 15 ± 3.71 mm) that healed completely after (median ± SD; 23 ± 3.99 day). The reduction in the lesion diameter accompanied with a disappearance of ulceration, pain and tenderness at the lesion site which is achieved after complete healing that obtained within 1 month of treatment.
TablE 2. The differences between lesion diameter before and after receiving treatment.
| Parameter | Treatment | N | Mean | Median | Std. deviation | Std. error mean | P |
|---|---|---|---|---|---|---|---|
| Lesion diameter (mm) | Before treatment | 50 | 20 | 20 | 4.93 | 0.7 | <0.001 |
| After treatment | 50 | 14.86 | 15 | 3.71 | 0.53 |
As demonstrated in Table 3, the diameter of LCL lesion before treatment, after receiving treatment, age, healing time and the number of visits required to achieve the intended outcome were all showed a strong significant and positive correlation with p-values less than 0.001.
TablE 3. The correlation between the diameter of lesion before receiving treatment, after receiving treatment, age, healing time and the number of visits.
| Number of visits | Time for healing (day) | Initial lesion diameter | Final lesion diameter | ||
|---|---|---|---|---|---|
| Age | r | 0.719** | 0.765** | 0.785** | 0.793** |
| p | <0.001 | <0.001 | <0.001 | <0.001 | |
| Number of visits | r | – | 0.942** | 0.819** | 0.818** |
| p | – | <0.001 | <0.001 | <0.001 | |
| Time for healing (day) | r | – | – | 0.826** | 0.829** |
| p | – | – | <0.001 | <0.001 | |
| Initial lesion diameter | r | – | – | – | 0.995** |
| p | – | – | – | <0.001 | |
**Correlation is significant at the 0.01 level.
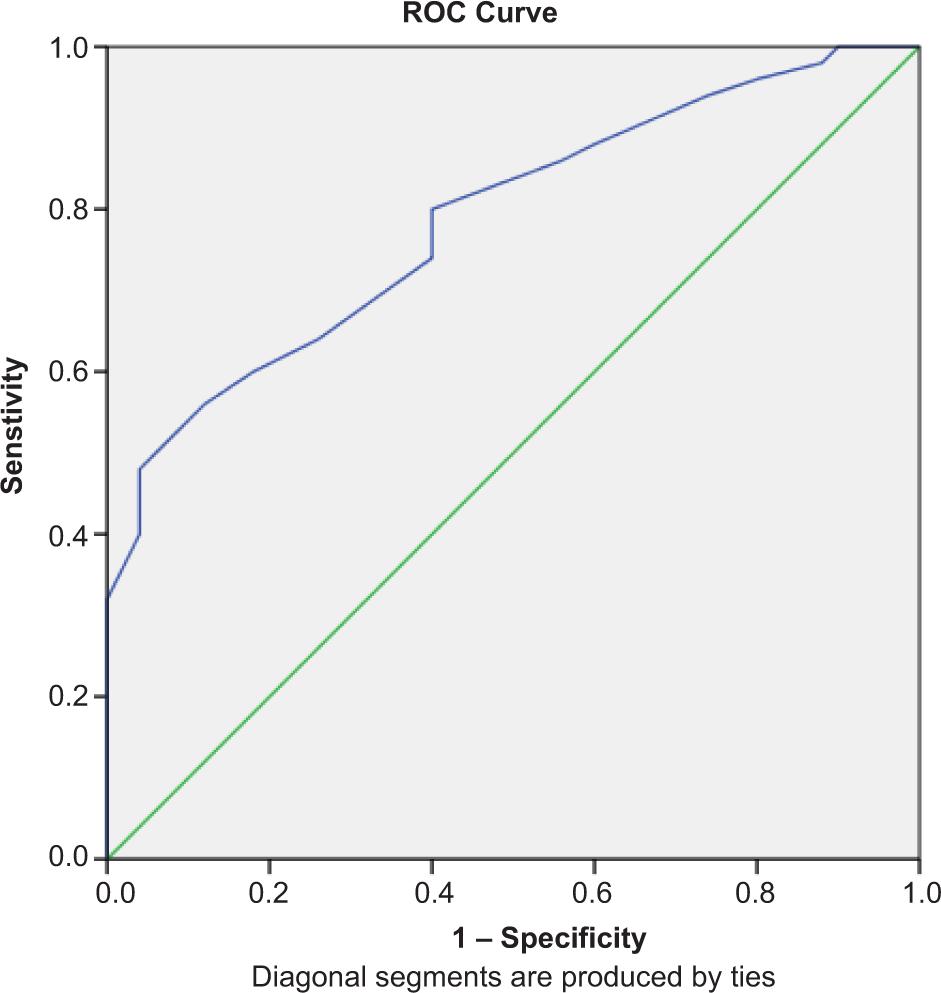
Receiver operating characteristics (ROC) curve results presented in Figure 2 revealed that the area under the curve is 0.786 with a sensitivity of 80% at a specificity of 60% for the use of intralesional levofloxacin in the treatment of LCL
FIG. 2. Receiver operating characteristics curve of the treatment.
DISCUSSION
Sodium stibogluconate is the first-choice therapy for CL treatment in Iraq, but in this study, intralesional levofloxacin was used for the treatment of LCL due to low availability and high cost of sodium stibogluconate.
Leishmania spp. lack organelles like mitochondria but have obtained a plastid via endosymbiosis from a green alga. The apicoplast is a nonphotosynthetic plastid that encloses numerous crucial metabolic pathways, making it sensitive to disruption from outside interactions. Elimination of the plastid or the complete suppression of its activity leads to “delayed death,” that is, the parasites grow and evade properly inside and from the first host cell, but their reproduction ceases upon invasion of the second host cell. DNA gyrase of the bacterial type and a circular DNA are both found in the apicoplast.10 It was known that levofloxacin inhibits bacterial DNA gyrase and topoisomerase IV.13 Therefore, the key finding of this study is the effectiveness of intralesional levofloxacin in the management of LCL. This is achieved due to the ability of levofloxacin to inhibit bacterial DNA gyrase in apicoplast that, which is found in Leishmania spp14–16 that is responsible for a number of vital biosynthetic pathways.17–19
In the present study, the results obtained support the above-mentioned hypothesis, in which levofloxacin have an antiparasitic action against CL as it showed a positive response and complete healing in 45 out of 50 patients who received the intralesional levofloxacin with a cure rate of 90% accompanied with a significant reduction in the diameter of lesion after 3–5 intralesional levofloxacin injection as demonstrated in Table 2. Complete healing of cutaneous lesion occurs within 1 month for all patients treated with the intralesional levofloxacin, which is significantly shorter than the duration required for self-healing of the lesion that is normally between 3 and 18 months.20
On the other hand, the results obtained in the current research revealed that there were nonsignificant differences between male and female in the diameter of lesion before and after receiving the treatment and also nonsignificant differences in age, healing time, and in the number of doses required to achieve the intended outcome (Table 1), which means that the gender may have no effect on the size of the lesion and also on the time and degree of improvement after receiving treatment.
Another interesting finding in the present work was the highly significant positive correlations between the measured parameters as illustrated in Table 3, which indicated that the initial size of the lesion affect the time of healing, in which the lesion with bigger size required more number of doses and takes longer time to heal completely. It is observed that all the parameters in this study are proportionally affected by age, where older patients showed larger lesion and require longer time for healing with more intralesional doses of levofloxacin. These results are compatible with the results presented by Oliveira et al. in 2011. In the study by Oliveira et al., it was found that the time taken for an ulcer to heal and the patient’s age were positively connected with the ulcer’s size at the time of the first clinical examination, which meant that a single measurement of the ulcer’s size during the initial medical visit served as a predictor for the time required to treat an ulcer.21
The study results showed that about 10% of the patients who received the treatment failed to heal completely, which may be caused either by low adherence to treatment and instructions (follow-up for at least three appointments for intralesional treatment, avoid humidity) or other contributing factors such as the racial differences and the specific features of the genetic variants.22–24 Another cause of treatment failure is the sensitivity of the affected area, when sensitive areas such as the eyelids, nasal region, and forehead are infected, The risk of failure will be increased by intralesional injections due to the restricted amount that can be injected, which restricts the dose to less than 0.25 mL for each centimeter diameter (0.25 ml/cm), which is below the required amount for optimum cure rate. Furthermore, a significant impact on the treatment failure was observed in multiple lesions compared to singular, which is documented by several studies that assumed that the rate of failure increases with each extra skin lesion. Multiple lesions might be an indication of high parasite load which prevent leishmania clearance.25
Moreover, ROC curve results that assess the reliability of using intralesional levofloxacin for the treatment of LCL revealed that AUC value were acceptable with good sensitivity and fair specificity that prove the previously presented data regarding the role of levofloxacin in the treatment of LCL.
CONCLUSION
Intralesional levofloxacin may have an important role in the treatment of LCL as it shows high effectiveness due to its antiparasitic activity and according to these results it can be considered as a promising treatment for LCL.
RECOMMENDATIONS
It is recommended to enlarge the sample size in future by subjecting more patients to such treatment in an attempt to prove the outcome that obtained in this research, given that the treatment used in this work is more available and cheaper than the traditional treatment used in LCL.
ETHICAL APPROVAL
The study has been approved by the Institutional Review Board (IRB) of the Department of Pharmacy, Kut University College, Wasit, Iraq.