BACKGROUND
The incidence of fungal infections has been increasing rapidly over the last few decades, with oral thrush or candidiasis being the most common. These infections are known to happen in individuals above 20 years and are regularly observed in older adults.1 Moreover, the incidence of oral carriage of Candida organisms is 30–45% in the healthy adult population. Candida, such as Candida albicans, Candida glabrata, Candida krusei, and Candida tropicalis, has been implicated as a causative factor in developing oral candidiasis. However, the incidence of Candida albicans is around 45–90% owing to various etiological factors.2 Some of the local and systemic factors, such as salivary gland dysfunction, dental prostheses, use of topical or inhalation corticosteroids, smoking habits, use of systemic drugs, an association of endocrine disorders, immunodeficiency conditions, and malignant diseases, have been associated with the predisposing factors for oral candidiasis.3 Oral Candida infection is generally present in acute or chronic forms. The main variants are pseudomembranous, erythematous, hyperplastic types, Candida-associated lesions, and keratinized lesions, which are superimposed with Candida.4 There are various mechanisms involved with the causative factors of oral candidiasis. The long-term use of topical and systemic corticosteroids is the treatment of choice in managing chronic inflammatory mucosal diseases such as oral lichen planus (OLP). Yet, the prolonged steroid therapy often necessitates antifungal therapy. The incorrect use of corticosteroids may lead to the suppression of cellular immunity and phagocytosis.
Nevertheless, this is a reversible phenomenon, as discontinuation of any corticosteroid form could improve local mucosal immunity. The alterations in the oral environment due to the long-term use of corticosteroids give rise to oral candidiasis.5 The diagnosis of oral candidiasis can be done using simple procedures such as identifying clinical signs and symptoms, a smear examination to determine the Candida hyphae in the epithelium, and serological tests.6 The local and systemic treatments using some antifungal agents such as polyenes azoles and natural anti-yeast substances have been the mainstay for treating oral candidiasis.1 In addition, systemic antifungals have been mainly indicated in disseminated disease and immunocompromised patients. Some antifungal agents, such as echinocandins, flucytosine, and posaconazole, have been known to inhibit beta-glucan synthase. They are widely employed in the treatment of oral candidiasis.7 There has been extensive research on natural anti-yeast substances such as berberine, grapefruit, garlic, cinnamon, xylitol, and volatile oil preparations, which are used as alternatives to minimize the side effects of conventional antifungal drugs.8 The prospective clinical studies have focused on developing a vaccine against Candida (live attenuated) SAP gene family proteins and glycoconjugates (Mannan & beta-glucan). However, the clinical trials are still fragmentary.1
ETIOLOGY, PATHOGENESIS, CLINICAL FEATURES, AND TREATMENT OF ORAL LICHEN PLANUS
OLP is a chronic inflammatory disease that commonly affects the skin and mucous membranes of the oral cavity.9 It is frequently seen in women, and a high prevalence of OLP has been noticed in smokers and alcohol abusers. It is also known to be triggered primarily by a genetic dysfunction and various environmental factors. Moreover, it has been associated with systemic diseases like hypertension, Diabetes Mellitus, Graft vs Host disease, and thyroid dysfunctions.9 The pathogenesis of OLP has been adequately investigated in the literature, where the T-cell mediated response and the auto-cytotoxic CD8+ cells are known to trigger apoptosis of basal cells. The cell-mediated immune mechanism starts with keratinocytes antigen, and this expression is followed by the migration of T lymphocytes and binding of antigen to (MHC 1) through activated CD4+ lymphocytes. The auto-cytotoxic CD8+ cells thus kill the basal cells of the oral epithelium through TNF-α.10,11
In 2003, the WHO made clinical modifications differentiating the OLP and oral lichenoid reactions. The OLP lesions are known to occur in various forms, such as bullous/plaque/papular, atrophic, erosive, and reticular, with the most common being reticular and erosive forms.12 The diagnosis of OLP has always been a big challenge for clinicians. However, a precise final diagnosis, through history and clinical examination supported by histopathological features and immunofluorescence testing, remains the standard diagnosis protocol.13 The differential diagnosis of OLP has been a critical tool in differentiating some of the oral lesions, such as lupus erythematosus especially (DLE), chronic ulcerative stomatitis, and pemphigoid-associated lichen planus. Due to the overlapping histopathological features along with clinical diagnosis further complicate the diagnosis of these cases.14 Furthermore, there has been a debate amongst clinicians on the development of candidiasis in the OLP lesions, especially with the erosive form, which further causes exacerbation of symptoms.
Moreover, there is no appropriate treatment for OLP, as there is a lack of complete curative treatment due to its recalcitrant nature. Symptomatic therapy has been widely recommended, such as applying lidocaine (2%) and benzydamine hydrochloride (0.15%). Soluble prednisolone tablets, betamethasone, fluticasone propionate, and clobetasol propionate ointment are also prescribed. Systemic steroids intramucosal injections with the administration of prednisolone and calcineurin inhibitors (cyclosporine, tacrolimus, mycophenolate), pre-con humanized monoclonal antibody therapy (efalizumab), Dapsone, PUVA, and photodynamic therapy have been established as treatment protocols for OLP.15
Candida in OLP Following Topical Steroid Therapy
OLP is usually present in six patterns: reticular, papular, plaque, erosive, atrophic, and bullous. Usually, erosive forms are more symptomatic and superimposed with oral Candida owing to mucosal soreness and pain.16,17 Oral candidiasis is known to exacerbate the clinical features of OLP, leading to symptoms such as a burning sensation or pain. Moreover, there is no established standard regimen for the use of antifungal drugs that prevent the development of candidiasis during the use of corticosteroids in the treatment of OLP.18 Types of topical steroids, such as clobetasol propionate, dexamethasone, fluocinonide, betamethasone, etc., have been widely used in treating OLP. Among these prescribed topical steroids, clobetasol has shown a significantly high rate of improvement in OLP, although the incidence of fungal infection was higher in these subjects. The reason is an increase in dosage, hence the prevalence of oral fungal infection.
Furthermore, among the various types of OLP, the reticular pattern has less fungal infection incidence than other types of OLP. The difference may be attributed to steroid agents, application type, and concurrent antifungal regimen use.19 In a study by Marable et al.,19 a multicentric retrospective trial was done to determine whether oral candidiasis can be prevented in OLP patients by using antifungal therapy. Antimycotic agents such as clotrimazole, chlorohexidine, probiotics-containing yogurt, nystatin, and fluconazole were used in this trial. The oral fungal infection was diagnosed based on clinical signs and symptoms and the presence of hyphae in the cytological smear. There was no difference observed in the improvement of OLP in patients treated with and without antimycotics.
Several studies have reported that patients with OLP likely to have positive Candida spp (Tables 1 and 2). cultures with blastophores, hyphal or pseudo hyphal cells, and a high-level count of Candida spp. in saliva (>1000 colony forming unit CFU/mL/mL) has been reported. Moreover, the growth of Candida upon topical steroids is attributed to enhancing the presence of active budding spores and hyphae, as noticed on cytological smears. Also, the use of corticosteroids is known to suppress the nonspecific inflammatory response and cell-mediated immunity, explicitly concerning T lymphocytes.20 The overall incidence of oral fungal infections upon topical steroids is between 11.4 and 76.7%, as reported by various authors.21–23 This wide variation likely reflects differences in diagnostic or sampling techniques and the use of cytological smear demonstrating at least one candidal true hyphal or pseudohyphal cell. Hyperkeratosis, a histological feature of OLP, may also predispose to Candida colonization and superficial erosion, as seen in OLP lesions.23 The risk of oral fungal infection development also depends on the application of corticosteroids, whether damp cotton rolls are placed at the affected site for 10 min or the corticosteroid is directly applied onto the affected area. However, Eisen et al. concluded that this made no difference.24 As reported in the previous studies, the OLP patients when treated with clobetasol had improvement in lesions, although patients treated with a combination of clobetasol and antifungal therapy had significant improvement. It may be due to the superficial fungal infection seen at the baseline, and as it was supplemented with an antimycotic agent, there was more improvement in the symptoms. There is no established standard for the delivery method of topical steroids and concurrent use of antifungal regimens. Clinical experience and training while treating such cases depend on the carefully chosen protocol varying with each clinician. Neglecting a potential candida infection might render the treatment of OLP ineffective. Thus, most clinicians may treat the OLP lesions with a combination of corticosteroids and antifungal therapy.22
TABLE 1. Summary of various reported studies implicating Candida in oral lichen planus (OLP).
| Author and year | OLP cases | Positive cases of Candida (%) |
|---|---|---|
| Jainkittivong22 | 30 | 23 (76.7) |
| Zeng et al.31 | 300 | 86 (28.67) |
| Mehdipour32 | 21 | 19 (80) |
| Masaki et al.33 | 15 | 12 (80) |
| Shivandappa34 | 34 | 15 (44.11) |
| Artico35 | 34 | 15 (44.11) |
| Werneck36 | 21 | 12 (57.14) |
| Arora et al.37 | 80 | 26 (33.3) |
| Hong He38 | 149 | 28 (18.87) |
| Miranda et al.39 | 84 | 21 (25) |
TABLE 2. Different schools of thought by various authors regarding oral candidiasis associated with patients diagnosed with OLP and the use of corticosteroids.
| Author | Year | Different schools’ thought regarding OLP and corticosteroids in association with Oral Candidiasis |
|---|---|---|
| De rossi and Ciarocca18 | 2005 | The use of topical steroids for OLP alters modulated local immune response, which implicates the cause of oral candidiasis. |
| Al Hashimi40 | 2007 | The use of topical steroids is considered a side effect or cause of oral candidiasis. |
| Lodi et al.21 | 2007 | Oral candidiasis is caused secondary to immunosuppression to treatment with corticosteroids. |
| Jainkittivong22 | 2007 | Spores and hyphae have evidenced the growth of Candida in OLP upon the use of corticosteroids on cytological smears. |
| Kragelund41 | 2013 | Erythema or superficial erosion in OLP lesions is a manifestation of the oral candidiasis. |
| Zeng31 | 2008 | There is an increased colonization of Candida in the erosive form of OLP. |
| Lundstrom42 Jainkittivong22 |
1984 2007 | Among the detected species of Candida, especially in erosive OLP, C. albicans has been highly reported. |
| Pfaller43 | 2012 | Antifungals resistance has been reported in patients diagnosed with OLP due to repeated use. |
| Lockhart44 | 1999 | Etiological factors such as immunosuppression, xerostomia, denture wearers along with OLP have been more prone to oral Candida infection. |
| Rautemma and Ramage3 | 2011 | Diabetes mellitus, along with diagnosed OLP, is a more prevalent candidate for oral candidiasis. |
| Miranda39 | 2020 | His research findings concluded that candidiasis alters the pattern of OLP lesions, with exacerbation of areas, and diminishes after the antifungal regimen has been taken. |
| Hong He38 | 2020 | The electrophoresis analysis has revealed that healthy controls had only one band, and multiple bands were seen in erosive lichen planus. Hence, Candida is more seen in erosive forms of OLP, which is similar to other studies. |
OLP, Oral Lichen planus.
Corticosteroid Use as an Implicated Factor for oral candidiasis inhalation corticosteroids
Oral candidiasis has been associated with regularly inhaled corticosteroids (ICS) to treat chronic obstructive pulmonary disease (COPD). They are usually prescribed with long-acting β2-agonists (LABA’SLABA’S). However, the severity of oral thrush depends on the type of inhalation of corticosteroid, the dosage, and the delivery device, such as a dry powder inhaler or a pressurized metered-dose inhaler. Drugs such as budesonide/formoterol propionate/salmeterol, and xinafoate/beclomethasone dipropionate have been administered for ICS.25 Several studies have reported that the incidence of oral thrush was higher in patients taking high daily doses of ICS compared to those taking low doses. The oral thrush is almost 85% higher when a high drug dose is prescribed.26 There is also the incidence of oral thrush with the use of spacers, proper use of it and the placing technique would further help reduce oral thrush.27 The synergistic effect of oral thrush has also been reported in patients with COPD, Diabetes Mellitus, and those on ICS therapy. The incidence of oral thrush in patients using ICS can be reduced using spacers, spacer devices for COPD patients with inhaler techniques, and inhaler therapy.28 Similarly, most patients taking systemic steroids may develop oral thrush upon early cessation; long-term use of corticosteroids has been implicated to have a low risk of candidiasis. It has been reported that glucocorticosteroid-induced biological effects cause increased susceptibility to fungal infections, which also influence lymphocytes, neutrophils, and eosinophils and directly affect Candida growth, morphogenesis, and virulence.29
Prevalence of oral candidiasis in Patients Diagnosed with OLP
A dispute among clinicians about whether OLP have been associated with oral candidiasis, the condition that further exacerbates the symptoms, and what treatment protocol has to be followed is still a debate (Tables 1 and 2).In OLP patients, the oral candidiasis prevalence rate ranges between 7.7 and 16.6%, as established through biopsy findings, whereas 37–50% has been noticed in culture findings, despite using corticosteroids and antifungal drugs as a treatment regimen.30 The species of Candida, especially C. albicans, adheres to the surface of oral mucosa and triggers inflammation which exacerbates the OLP. The enzymatic virulence of phospholipase is known to cause more significant toxicity.31 The OLPOLP types, especially the erosive, atrophic, and bullous forms of OLP, are usually erythematous, very symptomatic, and are often superimposed with candidiasis. In contrast, the reticular formation is typically asymptomatic and not imposed with a Candida infection. Hence, most of the research on Candida imposed on patients in OLP is seen in the erosive type of OLP.
RESULTS
Research Process According to PRISMA Guidelines. Data Search Programs
According to the results of this study, there is a positive correlation between the presence of oral candidiasis in the OLP’s patients treated with corticosteroids. Conferring to the analysis’ findings, the occurrence of Candida species in the cases with OLP is higher in the percentile in comparison to the control groups. The meta-analysis shows that oral candidiasis was associated with the range of 11–47% of the previous studies (Figures 2 and 3)
FIG 1. The meta-analysis was done for the studies to be included for the prevalence of the Candida species in patients with OLP.
FIG 2. The analysis results suggest that the occurrence of candida species in the cases with oral lichen planus is higher in percentile compared to the control groups in all the related studies.22,31–33,35–39,42
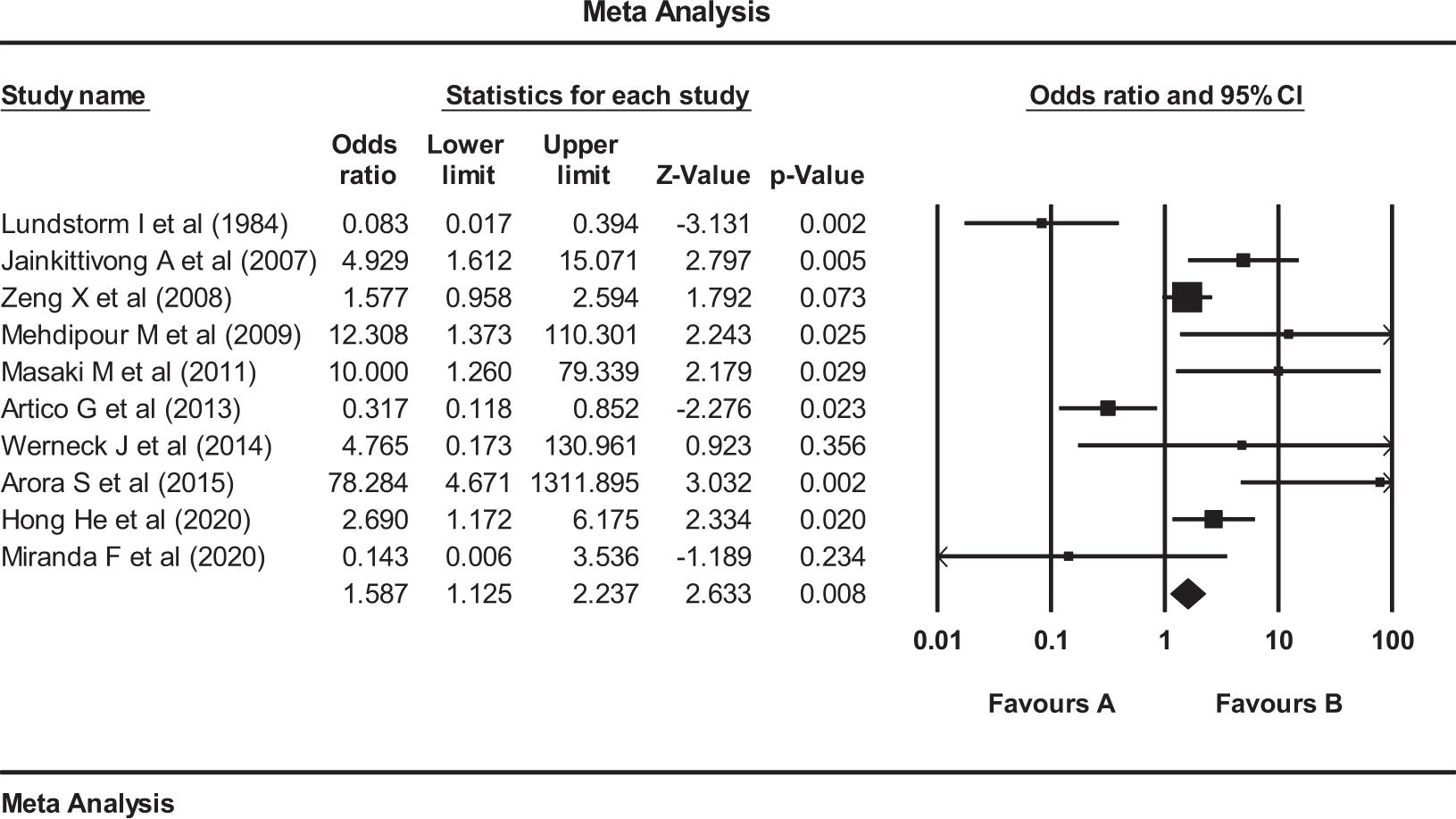
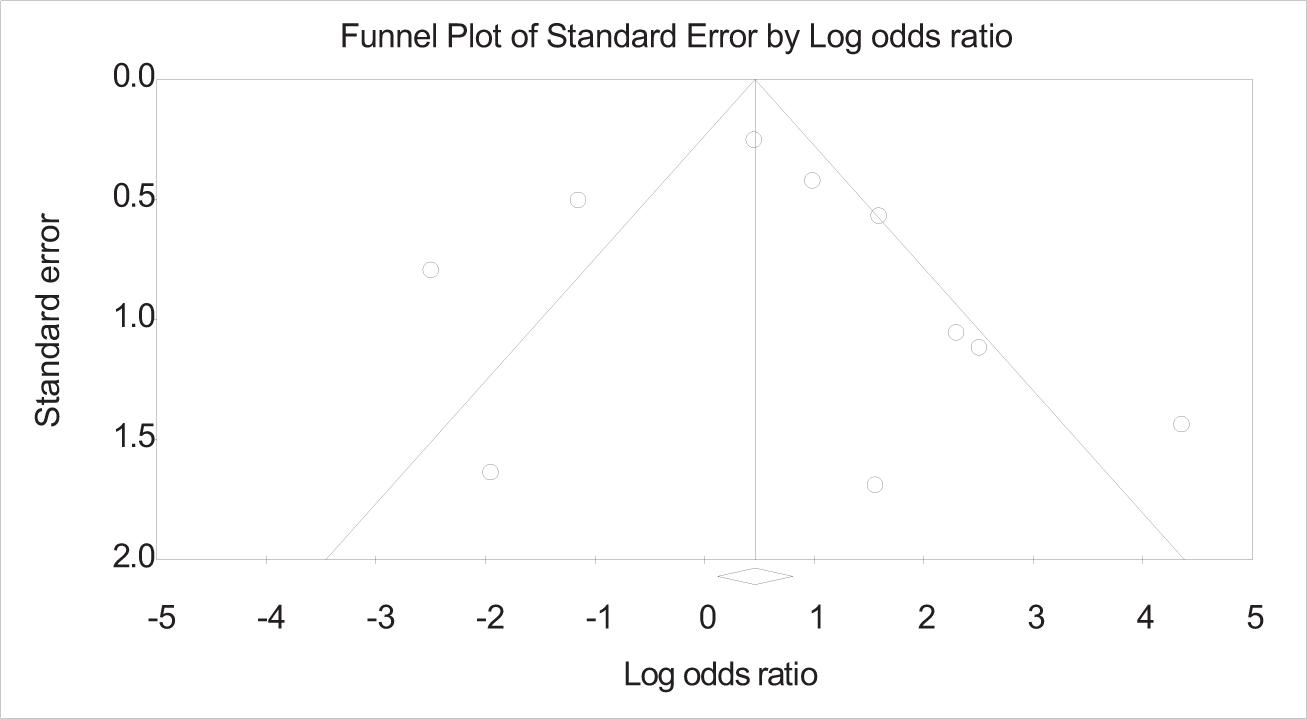
FIG 3. The funnel plot explains the publication bias at a low level, indicating that the authors included a positively correlated presence of Candida species in oral lichen planus. The odds ratio suggests that the Candida species is positively correlated with the lichen planus patients and is always on the positive side, indicating that patients with lichen planus are more prone to have a Candida infection.
DISCUSSION
This study investigates the association of oral candidiasis with OLP. Considering that oral medicine specialists, dentists, and general practitioners have shown increased interest in the link between chronic Candida infection and oral mucosal disorders, particularly OLP and oral leukoplakia, this analysis focuses on OLP. The purpose was to highlight the relationship between Candida and patients diagnosed with OLP, to correlate the use of steroid medication, and to identify the factors that may contribute to oral Candidiasis. Long-term administration of topical and systemic corticosteroids is meant to safeguard against the need for antifungal therapy in treating chronic inflammatory mucosal illnesses such as OLP, a persistent inflammatory condition, frequently impacts the skin and mucous membranes. This condition has been more prevalent in smokers and drinkers and is commonly encountered in women. It is also recognized that a genetic malfunction and several environmental circumstances also play a significant role in its onset.
Additionally, the condition has been linked to systemic illnesses such as thyroid dysfunction, hypertension, Diabetes Mellitus, and Graft vs Host disease. Most research has concentrated on the pathophysiology of OLP. On that note, there are two types of oral Candida infection: acute and chronic conditions, with the main variants being: pseudomembranous and erythematous, the hyperplastic varieties are associated with OLP, and the keratinized type is associated with OLP as well.
A new microbiological assessment of OLP supports the concept that this inflammatory illness might create favorable conditions for bacteria colonization.39 According to Zeng et al. and Masaki et al., OLP may predispose to specific Candida genotypes As previously observed in most OLP studies, majority of the patients were primarily investigated for burning sensation in the first 66 months since the appearance of the lesions. The buccal mucosa was the most frequently affected site of the oral mucosa, which is in concordance with previously reported studies.
In the present meta-analysis, we concluded that oral Candidiasis was associated with in the range of previous studies, 11–47%. Adding oral Candidiasis to the OLP lesions was established to significantly exacerbate the symptoms as well as the clinical features.
In atrophic and erosive forms of OLP, the manifestation of candidiasis aggravates the lesions, primarily associated with the inflammatory response resulting from the adherence to the oral mucosa. Such cases were also encountered in our clinical experience. But identifying Candida species is essential for successful therapy selection. As OLP has a chronic progression, in most cases, complete clinical remission is difficult even under treatment. The most commonly utilized standard topical remedy encompasses a moderate corticosteroid integrated into Orabase. Nevertheless, this favors the growth of Candida and ultimately leads to a relapse of oral Candidiasis. The formerly tallied incidence rate of oral Candidiasis, quoted at 13.6% in treating OLP patients using steroids, stresses the significance of antifungal adjuvant treatment.37 Thus, it is critical to individualize the antifungal use, frequency of topical applications, and periods of OLP treatment for each patient based on their general health status and the severity of the lesions they present with. The relationship between varied physiological conditions or widespread diseases justifies the necessity for inferences from related health specialties. For example, avoiding treatment for OLP during pregnancy is recommended. Still, pregnant women may accept treatment in severe erosive cases based on previous discussions with an obstetrician to evaluate risks to the fetus.36
Besides oral candidiasis, dental foci and inadequate oral hygiene are local factors that could contribute to a prolonged healing of OLP lesions. Furthermore, even though probiotic bacteria were quoted as reducing the carriage of oral Candida in vivo in the OLP lesions, the administration of the treatment showed no significant reduction in oral Candidiasis recurrence.38
On the other hand, Miranda et al. assessed whether Candidiasis influences the clinical behavior of OLP lesions in 32 participants.39 Their analysis revealed that Candidiasis alters the pattern of OLP lesions, with worsening areas, and diminishes after the antifungal regimen has been taken. Moreover, the intake of corticosteroids used in treating OLP is suitable for developing oral Candidiasis. Various authors state different schools of thought regarding the association of oral Candida in OLP patients. Shivandappa et al., have noted that the fungal infections in OLP have consistently been underestimated, and it presents as a subclinical infection detected in biopsy or microscopic examination.45
Similarly, Krogh et al. stated that the ability of Candida to colonize in OLP patients could be due to an imbalance between Candida albicans virulence factors and host defenses, or it could be due to defects in the immune system.45 Likewise, Lodie et al. have researched the controversial aspects of OLP associated with candidiasis and stated that Candida exacerbates the OLP lesions.21 Hence, if antifungal agents are administered, there could be decreased potential of Candida albicans to produce carcinogens such as N-nitroso benzyl-methyl-amine. As noted, the erosive lichen planus has more malignant potential. Gayathri et al. have confirmed that specific surface proteins called adhesins help adhere to the cell surface bringing specific phenotypic changes from yeast to hyphae forms, thus producing carcinogenic compounds such as nitrosamines and N-nitroso benzyl methylamine.46 These carcinogens by nitrosation often lead to precancerous changes. The candidal yeast cells thus extend from the mucosal surfaces to the deeper layers of oral epithelium and deposit nitrosamines. Therefore, dysplastic changes are evident in patients of erosive lichen planus in particular.
Similarly, Lundstrom et al. reported that the low secretion of unstimulated saliva might also lead to the development of Candidiasis in OLP patients owing to the alteration of pH and long-term use of corticosteroids.42 Likewise, Hatchuel et al. have reported that Candida infection mainly occurs in OLP and nonspecific lichenoid stomatitis without ulceration.30 Also, Hong et al. concluded that specific genotypes of Candida albicans might probably involve the progression of OLP.38 In the same way, Simark Mattson et al. have proved that the inflammatory potential, that is, the proliferation of cytokines and peripheral mononuclear cells, was reduced by the stimulation of Candida albicans, thus reflecting the immune regulating mechanism of OLP modulating Candida albicans.47 There has also been a controversy over the presence of OLP in Candida, whereas Artico et al. found that the colonization of Candida was more in healthy subjects than in OLP.35 The importance of genotyping in identifying the Candida species and the difference in phenotype has been tried by Hong et al., where their electrophoresis analysis has revealed that healthy controls had only one band, and multiple bands were seen in erosive lichen planus38 (E-OLP). In addition, Zeng et al. have reported that OLP attracts Candida albicans, and exacerbation of lesions may be due to dental health problems and improper oral hygiene.31 Therefore, improving oral hygiene and solving all dental issues by adopting immune drugs and using herbal products may cure OLP-associated candidiasis.
Gainza-cirauqui has suggested that Candida albicans which have been isolated from carcinogenic oral lesions, produce acetaldehyde causing abnormal epithelial proliferation of mucosa.48 Clinicians in such cases should seek their attention to the presence of any premalignant dental to mucosal frictional injury while treating OLP patients. Marable et al. conducted their research to determine the incidence of oral candidiasis in patients treated with topical steroids for OLP.19 Their findings concluded that none of the preventive antifungal strategies have been effective in such cases.
Jain Kittivong et al. have investigated the presence of Candida in OLP patients using salivary and imprint cultures and those patients taking corticosteroids.22 Their findings suggest that topical steroids increase Candida growth with associated risk factors like age, medication, and use of dentures.
In the matter of the implication of corticosteroids for the development of oral Candidiasis, in a study by Marable et al., 315 patients with OLP were treated with topical steroids for 2 weeks.19 One group was treated with antifungals and the other group without antifungals. An incidence of 13.6% was seen with candid, and no significant difference reported in the rate of development of Candidiasis was observed with those treated with and without an antifungal regimen.
Cribier et al. have concluded upon their critical appraisal findings that the use of topical corticosteroids in the treatment of lichen planus has been beneficial.49 While secondary Candidiasis is a possibility upon the use of fluocinolone acetonide (0.1%), four applications per day for 4 weeks, systemic corticosteroids such as prednisolone 0.75 mg/kg/day for 3 weeks are used in the treatment of lichen planus, where secondary Candidiasis was observed. Henriksen et al. mentioned the side effects of ICS and systemic or topical antifungals.50 Out of 28,894 patients using inhaled ICS, 5089 persons were given antifungal therapy after 30 days of ICS, and 39 patients were given on the same day. The increased candidal growth was based on ICS inhaler type and dose, predominantly in males and in older age, as well as the particle size of ICS. Miravittles et al. researched to analyze the long-term adverse effect of corticosteroids in the treatment of COPD where they concluded that there is a 5.5% increase in oral Candidiasis in patients taking ICS, as the dose increases, there is a risk of fungal infection.51
CONCLUSION
The systematic review and the meta-analysis aimed at the different studies and their prejudice regarding the presence of oral candidiasis in OLP patients treated with corticosteroids. The purpose of this meta-analysis was to highlight the relationship between Candida and patients diagnosed with OLP, to correlate the use of steroid medication, and to list the factors that may contribute to oral Candidiasis. The meta-analysis concluded that there is a positive correlation between the presence of Candida species in them. Thus, they are more prone to developing Candidiasis and have to be looked upon with additional antifungal therapy and corticosteroids.
Over the past few decades, the prevalence of fungal infections has been rising quickly, with oral thrush or Candidiasis being the most prevalent. In the population of healthy adults, oral candida organisms are carried by around 30–45% of people. Oral Candidiasis has been linked to the presence of Candida species such as Candida albicans, Candida glabrata, Candida krusei, and Candida tropicalis. However, due to many etiological factors, Candida albicans is identified to report between 45% and 90% of cases. Oral Candidiasis has been linked to several local and systemic factors, including salivary gland dysfunction, dental prostheses, use of topical or ICS, smoking, use of systemic medications, an association of endocrine disorders, immunodeficiency conditions, and malignant diseases.
According to the analysis’ findings, the occurrence of Candida species in the cases with OLP is higher in the percentile in comparison to the control groups. The meta-analysis shows that oral Candidiasis was associated with the range of 11–47% of previous studies. Notably, oral Candidiasis added to the OLP lesions caused an aggravation of the symptoms and the clinical features. As such, the different studies reviewed here show the presence of oral Candidiasis in the OLP patients treated with corticosteroids, which, in turn, concludes a positive correlation between the presence of Candida species in the patients. However, in ideal future studies, a diagnostic approach for oral fungal infections and OLP can be established through a baseline assessment for Candida spp. carriage, identification, susceptibility, and subjective and objective outcome measures. The futuristic approach of personalized treatment would further help clinicians make decisions. For example, before initializing therapy, assays for Candida species can be performed, and where there are low CFU counts, mouthwashes such as chlorhexidine or established herbal regimens can be selected. However, clinicians’ opinions vary as they may give corticosteroids unless there is recalcitrant nature of OLP or severe Candida infection.41 Moreover, a potent antifungal regimen should be implemented for those with high species of Candida and risk factors such as denture wearers, low salivary rate, radiation therapy or antibiotics, and tricyclic antidepressants. A future study would be needed to solve such a diagnostic dilemma, and whether the clinical features of OLP may be influenced by Candida colonization and challenges to such an approach need further investigations and conclusions from various researchers and clinicians.
AUTHORS’ CONTRIBUTIONS
All authors have read and approved the final manuscript.